So you're visiting Southeastern Arizona (or perhaps you live here) and you want to find as many species of psyllids as reasonably possible? Here's everything you need to know.
The Basics
First, it's good to remember that psyllids are small. Most species average 2-3mm, and most native species aren't super prolific on their hosts and can be hard to spot in the field. Some species may produce galls or other forms of leaf damage on their hosts, which can be useful to recognize. Some species may sporacidally come to lights, but sweeping or beating known host plants is usually the most effective way to find psyllids. It is recommended to familiarize yourself with the psyllid hostplants outlined here first (or just invite me with you on your psyllid expeditions I know where everything is).
Lowland species
Part 1: Mesquite Psyllids
As you're exiting your hotel or house and walking to your car you may notice that there are a lot of mesquite trees in Southern Arizona. You'll find mesquite in natural areas but also all over the cities planted along the streets and parking lots. If you look in any given direction from a random point in the city of Tucson you'll probably have a mesquite tree in your line of vision. Many different insects feed on mesquite, and you can probably spend days documenting the various species that you can find on the plant. You can reasonably expect to find 3 species of psyllids on the plant (shown below) and you'll probably be able to find many additional cool non-psyllid insects as well while you look for them.



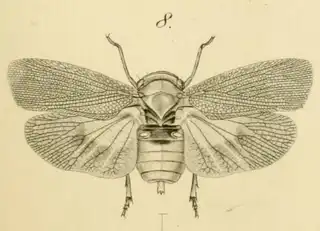
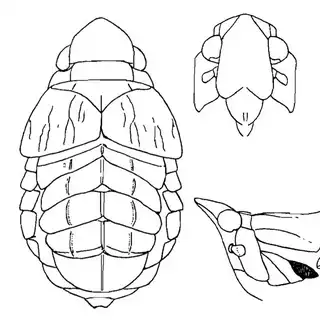
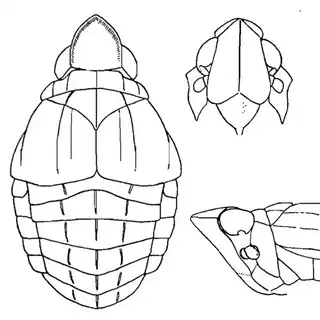
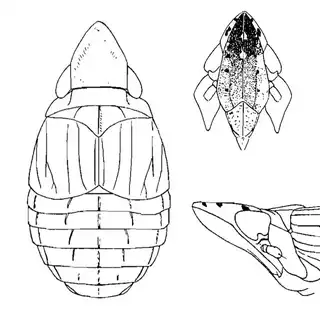
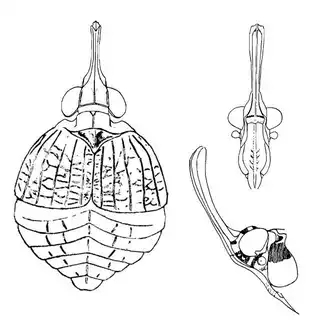
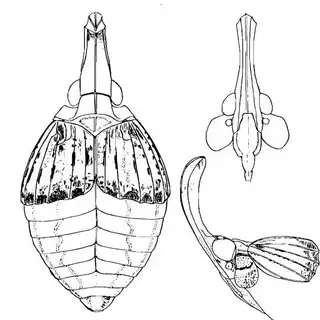
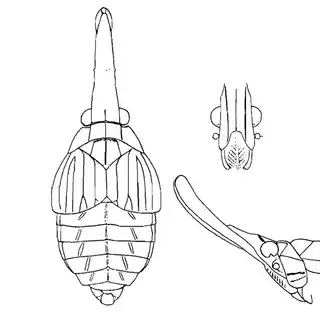
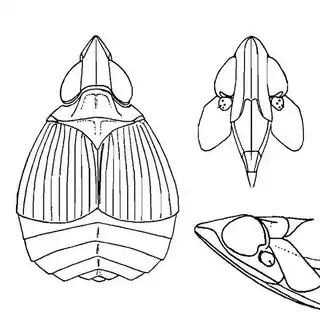
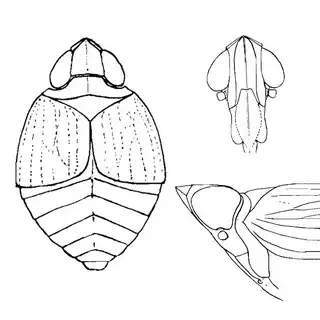
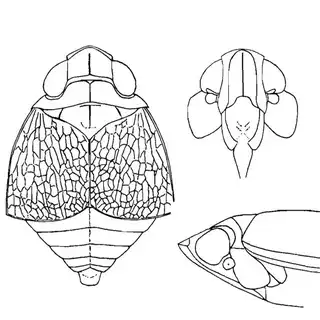
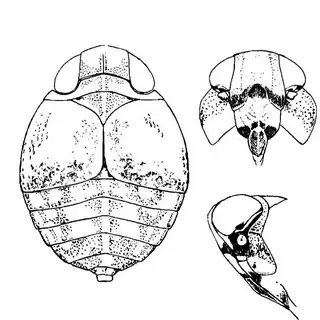
Heteropsylla texana (above, left) is fairly distinctive, with consistent dark maculae on the wing. The other two species, Aphalaroida inermis (above, middle) and Aphalaroida spinifera (above, right) are more variable, ranging from uniformly green to brownish with a well defined pattern, but the two species can always be told apart by the absense or presence of dense hairs covering the entire body and wings. While Heteropsylla texana and Aphalaroida inermis are found throughout the western states, Aphalaroida spinifera is an Arizona endemic, and fortunately not too hard to find.
Part 2: Some related species on other Mimosoid legumes
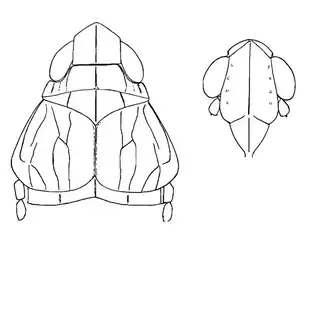
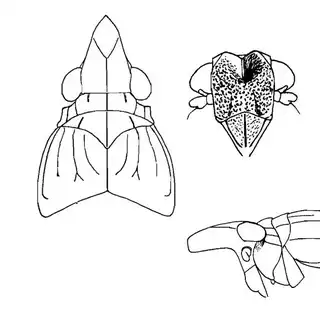
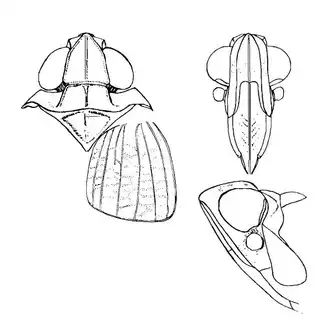
Mesquite is just one of several Mimosoid trees common in arid parts of southeast Arizona, and it's good to familiarize yourself with a few others. Thorn trees (Vachelia) and catclaw Acacia (Senegalia greggii) both host psyllid species, with Aphalaroida pithecolobia (below, left) on the latter and an undetermined Aphalaroida sp. (below, right) on the former. Both are similar to the aforementioned Aphalaroida spinifera but with generally shorter setae on the wings and body.


Part 3: Lycium Psyllids
Lycium (boxthorn) is a shrubby plant not quite as ubiquitous in cities as mesquite, but it is still very common in lowland areas and you shouldn't have difficulty finding it in canyons or other natural areas. 3 species of psyllids can be found on Lycium, and I guarantee you will find at least one.



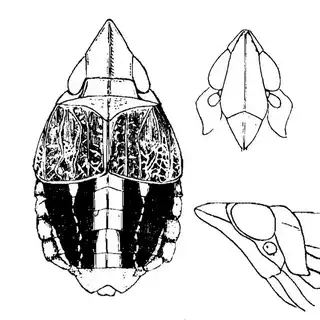
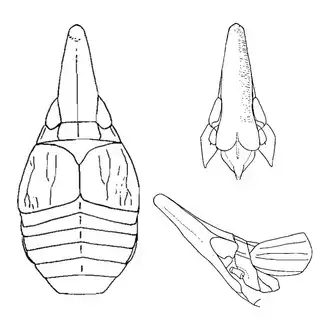
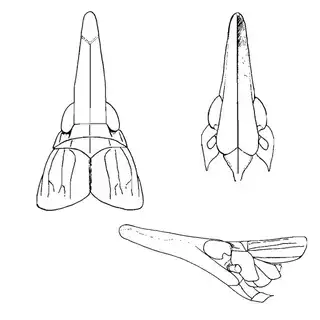
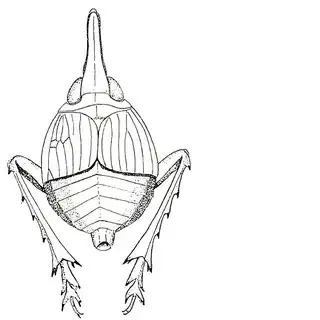
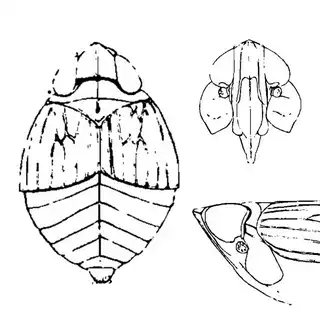
Bactericera cockerelli (above, left) is without a doubt the most common western species. While Lycium is its native host, it has expanded its range to include other Solanaceae such as cultivated potato, tomato, and peppers, and is often regarded as a pest because of this. You will likely be able to find this species on Lycium growing in Arizona, but it is also likely not going to be your primary target. Bactericera dorsalis (above, middle) is a much more interesting find, but at a glance it can easily be mistaken for the Bactericera cockerelli. The coloration is different but also quite variable, and the the wings are more angulate and the venation different; I advise looking through images to familiarize yourself with the extent of variation of this species so that you don't overlook it and mistake it for Bactericera cockerelli. The final Lycium species, Bactericera lobata, is the real main attraction though it is a much rarer find. The wings are heavily maculated and is unlikely to be confused with anything else.
Part 4: Hackberry Psyllids
If you're from the Eastern USA you might already be familiar with hackberry psyllids, many of which are gall-inducers and can be quite abundant. The hackberry psyllid fauna of Arizona is somewhat unique, and worth investigating even if you think you're familiar with this group. The two hackberry species you'll want to familiarize yourself with are desert hackberry (Celtis pallida) and netleaf hackberry (Celtis reticulata). The former is more common and can generally be found in more arid habitats, while you may have better luck finding the latter along streams. Desert hackberry hosts a single species of psyllid, Leuronota maculata (below), though it shouldn't be overlooked. The species can become quite abundant on its host and can often be seen swaying its abdomen from side to side.

Netleaf hackberry, in contrast, is host to many species of psyllids, most of which are gall-inducers. The galls are indeed the easiest way to identify psyllid presence on netleaf hackberry, though if you're lucky you may be able to find adults as well



On the petioles you will find large galls of Pachypsylla venusta (above, left), a common species throughout North America. On the buds on the stem you will find distinctly hairy white galls which belong to Pachypsylla pallida (above, middle), a rarer and primarily western species. And on the leaves you will find blister galls in extremely dense numbers, belonging to Pachypsylla celtidisvesicula (above, right). It is interesting to note that the nipple-gall maker Pachypsylla celtidismamma as well as other eastern leaf-gall species are entirely absent from southern Arizona; what this means is that adult Pachypsylla can be reliably identified to species level in this region, whereas they can't be in the eastern USA (as all adult leaf-gallers look identical). It might also be interesting to note that the both the blister galls and the blister gall psyllid adults of Arizona look slightly different than those found in the Eastern USA, but for the time being these are regarded as a single species. Future work may prove them to be distinct. The adults of the hairy bud gall psyllid (below, left) and blister gall psyllid (below, right) are easily distinguished and are shown below.


If you are very lucky you may find, in addition to the galls outlined above, small white lerps on hackberry leaves. These belong to the Sugarberry Psyllid Tetragonocephala flava, an attractive southern species. Both the lerps and adult are shown below.


Image credits: left: © Leonardo Hernández Escudero, some rights reserved (CC-BY-NC). Right: © Catherine C. Galley, some rights reserved (CC-BY-NC)
Part 5: Other species on miscellaneous low elevation plants
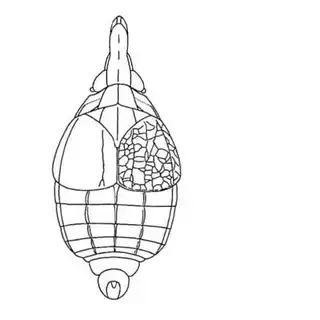
Ambrosia monogyra is a common plant along washes and it is very regularly densely covered with interesting floral-like galls (below, middle). The inducer of these galls remained a mystery until last year when I determined it to be the psyllid Craspedolepta lapsus (below, left), a species previously described from Texas. I found adults in autumn, but you may be able to find nymphs inside the galls in summer.



Calinda is another gall-inducing psyllid, responsible for flower galls on Baccharis. Unlike the previous species, the galls are difficult to spot, but sweeping the plants can often produce adults. On Desert Broom in particular you will find Calinda longistylus (above, right), but a few additional Calinda may be found in Arizona as well.
Mid and high-elevation species
One thing you might have noticed about Arizona is that it can get kind of hot, and lurking about the desert finding tiny bugs past noon isn't for everyone. Luckily, a number of interesting species can be found in the mountains after a successful morning of finding all of the aforementioned species.
Part 1: Rosewood psyllids
Arizona rosewood (Vauquelinia californica) is an interesting shrubby tree that you'll find at low to mid elevations in southern Arizona; in the Santa Catalina Mountains you shouldn't have trouble finding it growing by many pulloffs on the side of the highway, starting around Molino Canyon Vista and continuing up to the bend just before Bear Canyon. The psyllid fauna of this plant, previously unrecorded, is a subject of ongoing research by me. 2 species are confirmed for the host and a third is expected. All of the species belong to the Arizona endemic genus Metatrioza (the species Neotriozella hirsuta is currently misplaced based on unpublished data).


Left, Metatrioza neotriozella. Right, "Neotriozella" hirsuta
Part 2: Willow psyllids
In riparian situations you will find willows, and where there are willows there are psyllids. Willows (Salix) are the most commonly used host plant for psyllids in North America, with dozens of species associated with the plant. As such, identification can be difficult.



Goodding's Willow (Salix gooddingii) is the most commonly observed willow in southern Arizona, and on it you will find Bactericera minuta (above, left), a psyllid easily recognized by the narrow black dorsal margin of the wing. Both this host and its psyllid can be found at lower elevation riparian areas, but at higher elevations a much more diverse selection of willows yields a more diverse selection of psyllids. Bactericera californica (above, middle) is one such species, and fairly rare. Much more common are Cacopsylla spp. (above, right), a genus which includes many similar species which feed on willow. Adults of this genus are most common on their host March-May, after which they often migrate to coniferous trees to overwinter.
Part 3: Sumac psyllids
Sumacs (Rhus) are found throughout North America and are a typical host for the psyllid genus Calophya. Members of this genus are found throughout the country, with a few interesting species found in Arizona. Expect a full article from me detailing the Calophya of North America including Mexico very soon.


Evergreen sumac only becomes common in mountains south of Tucson, and on it occurs the psyllid Calophya minuta (above, left), a rare species known only from Arizona and Texas. The more common species of sumac throughout the rest of the state is Rhus aromatica, which is the host plant for at least 5 Calophya species in western North America. Of these, at least 2 have been observed in Arizona: Calophya aromatica, and a Calophya sp. nr. dubia (above, right, previously not recorded from the state). Future collecting and observing on this host plant may reveal the presence of additional species.
Part 4: Manzanita and Madrone psyllids
Over 60 species of manzanita are found in California, on which there are numerous psyllids, but Arizona isn't California. Nonetheless, Arizona's mid-elevation chaparral habitats have a healthy supply of mostly Pointleaf Manzanita (Arctostaphylos pungens), on which you may be able to find the common manzanita psyllid Neophyllura arctostaphyli (below, left). California's more unique manzanita psyllids appear to be absent from Arizona.


Madrone Psyllids belong to the same genus but are easily recognized by their highly sinuate wing veins and well, their preference for madrones. Three madrone psyllids are found in North America, all geographically distinct, with one species in the PNW, one species in Mexico, and finally one species in southeast Arizona. This species, Neophyllura arbuticola (above, right) is restricted to Arbutus arizonica. So far it is only known from the Chiricahuas, Huachucas, and Santa Ritas; attempts to find it in the Santa Catalina Mountains have been unsuccesful.
Part 5: Ceanothus psyllids
Ceanothus is an extremely diverse genus of plants in California and is host to over a dozen species of psyllids, but once again, Arizona isn't California. Only a fraction of California's Ceanothus and Ceanothus psyllid diversity crosses over into Arizona, however, Ceanothus psyllids are wonderful and Arizona's psyllid fauna does include one unique species. Fendler's Ceanothus is common in ponderosa pine forests at higher elevation and all psyllids detailed here were found on that plant, but studies of additional species of Ceanothus in Arizona may yield additional species of psyllids.


Ceanothia ceanothi is, as the name implies, the Ceanothus psyllid. It is the most common Ceanothus psyllid in western North America, and one of the plainer species. Ceanothia essigii (above, right), in contrast, has distinctly blackened wings, and until my discovery of the species in the Santa Catalina Mountains was considered a California endemic.


Euglyptoneura robusta (above, left) is a widespread but extremely beautiful species which is also found on Ceanothus. Nyctiphalerus propinquus (above, right) is another attractive species, and is essentially the sky island equivalent of the Californian species Nyctiphalerus vermiculosus. it is not unusual to find multiple species of Ceanothus psyllids living together on the same plants.
Part 6: Purshia and Cercocarpus psyllids
The subfamily Dryadoideae in the Rosaceae contains a couple of closely related plant genera, the cliffroses (Purshia) and the mountain mahoganies (Cercocarpus). In western North America these plants serve as hosts for a great many species of psyllids, most of which have now been referred to the genus Purshivora. Once again, the bulk of psyllid diversity surrounding these plants is endemic to California, but a number of species occur in Arizona as well and likely even more await discovery. In the Santa Catalina Mountains, look for these plants to start becoming more common beyond Windy Point.



On Cercocarpus you may find Purshivora insignita (above, left), a species whose presence may also be recognized by its distinctive leaf-curl galls. A similar species, Purshivora coryli (above, middle), can be found on Purshia. Cacopsylla nana (above, right) probably also belongs to the genus Purshivora, and I have found it on Cercocarpus. This species, unlike the others, is apparently an Arizona endemic, with a distinct forewing pattern that makes it easy to identify.
Part 7: Alder psyllids
As you reach the highest elevations you'll find massive alder trees, especially Arizona alder. Several species of psyllids are found on alder throughout the country, one of which, Psylla alni americana (below), is known from Arizona.

Rare Stuff
So you've found all of the above species and you want more? Here's a few additional species to keep your eyes open for, if you can find them.


So here's the thing: I've found Trioza mexicana (above, left) multiple times in the Santa Catalina Mountains, on various plants: Ribes pinetorium, mananita, box elder, douglas fir; however, none of these things are likely to be the host plant. All we really know for sure about it is that it is a high elevation species, observed from 7000-9000 feet, but not knowing the host plant makes it a very difficult species to track. On the other end of the spectrum is Heteropsylla intermedia (above, right). The hostplant of this species is known: Mimosa, apparently Mimosa aculeaticarpa in Arizona. Until recently this species was known only from Mexico, now also known from a single record from Arizona and a single record from Texas. I found the Arizona specimen at the Bug Spring Trailhead in the Santa Catalina Mountains - if you go looking for one, good luck!