A review of the literature on sclerotia in Leucocoprinus species and other Agaricales
Abstract
Whilst there are many fungi known to produce sclerotia, documentation of them in Leucocoprinus species is lacking with only a few species recorded as producing them and little information on each. As L. birnbaumii is one of the most common species to find in plant pots and has sclerotia which are readily visible owing to their size, colouration and vast numbers spreading across the top of the soil this may be suggestive of sclerotia facilitating transmission via potting soil. It seems probable that other Leucocoprinus species found in plant pots can also spread via sclerotia, but that they simply produce less visible sclerotia which go unreported. Culturing Leucocoprinus cretaceus in a sterile substrate has produced abundant sclerotia followed by mushrooms and sclerotia have also been found in cultures of L. cepistipes and L. cepistipes cf. var. rorulentus both from plant pots. This review was initially intended to research which Leucocoprinus species sclerotia had previously been documented in. However besides from Mattirolo's 1918 study there appears to be little documentation of sclerotia in Leucocoprinus species. Sclerotia are not documented in many Agaricales in general but studies of sclerotia in some Coprinopsis species reveal some universal traits that are applicable to other species. Minute sclerotia have subsequently been found stuck to the stipe of Conocybe cf. macrospora from a plant pot demonstrating how easily these structures could be overlooked if not specifically looking for them. This literature review is therefore presented in order to summarise information such that it may assist in future research into sclerotia.
1. Introduction
The presence of sclerotia in Leucocoprinus species appears to be an under-studied subject with little to no documentation of sclerotia in anything other than Leucocoprinus birnbaumii and only a small number of Agaricales documented as producing sclerotia.[1] The large number of observations of L. birnbaumii provides good evidence for how abundantly sclerotia are produced by this species as they are readily seen but it may not be so apparent with other species as they do not get documented and are not easily seen in observation photos. The yellow to whitish-beige sclerotia of L. birnbaumii are frequently visible on the top of soil in plant pots, in bags of compost or against the glass of terrariums and vivariums growing amongst the coir. At a little under 1mm in size (500-800µm),[2] these small, hard structures are easily seen with the naked eye and indeed frequently become noticed.
As these sclerotia are produced in such vast numbers and are of such a discreet size they could be easily distributed through potting soil when plants are repotted or propagated from root suckers. This may provide an explanation for how this species was introduced into greenhouses and plant pots from tropical plants brought back by early explorers and why it continues to be a common find in plant pots. Such structures could function similarly to resting spores allowing the species to lay dormant in soil until conditions are right to grow.
Houseplant blogs and forums are a good source for observations of these sclerotia because when people find them in their pots they tend not to know what they are looking at and so automatically worry that it may harm their plants and hence are inclined to seek advice online. Posts asking if they are mold, spider mite or insect eggs are common - as are people responding and diagnosing it as one of these things. The fear of the unknown is such that people will often panic and replace all their soil or dowse it in vinegar, hydrogen peroxide or even odder home remedies which seem unlikely to work and more likely to harm the plant than taking no action would.
Search engine image results are therefore full of observations of Leucocoprinus sclerotia misdiagnosed as something else entirely. Many people do however recognise them as a common fungus that is harmless for the plant and so reassurance usually comes, occasionally with an identification of them as L. birnbaumii but very rarely with the explanation that they are sclerotia. When they do get identified as L. birnbaumii, they're usually either dismissed as primordia, mycelium or no further description is provided. Despite sclerotia in L. birnbaumii being documented a long time ago and being commonly observed ever since it appears that information about them just has not been widely disseminated. Given the lack of awareness and scarcity of information on the sclerotia which are so readily visible in L. birnbaumii it is perhaps no surprise that there appears to be little information on sclerotia in other Leucocoprinus species. It may be that it is only via culturing these species that their sclerotia production can properly be documented and explored.
2. Taxonomic history of Leucocoprinus sclerotia and previous studies
Sclerotia of Leucocoprinus species appear to have been observed and described many times since the 1800s with some early classifications of them as fungi imperfecti belonging to an unknown species before observations of them were made with the associated mushrooms. The taxonomy on them however is confusing and filled with many erroneous entries and mistaken information, much of which is addressed by Oreste Mattirolo in his incredibly comprehensive 1918 text (in Italian).[3] This work appears to the be the most comprehensive study of Leucocoprinus sclerotia to date with much of the taxonomy addressed by Mattirolo and so the key points will be summarised here along with collating the other sources.
Paul Christoph Hennings may have been the first to describe the sclerotia in a Leucocoprinus species along with the associated mushrooms in 1898 when they were noted growing on soil in the hothouses of Heinrich Hildmann's cacti gardens in Birkenwerder, Germany. The early taxonomy of Leucocoprinus species however is just as confusing and conflated as that of the sclerotia and so Hennings attributed them to L. cepistipes, though described the mushrooms as golden yellow suggesting he was actually describing L. birnbaumii. This confusion is common owing to a number of early illustrations and descriptions which conflated several species or considered them all the same, only varying in colour. The sclerotia are described as small, barely 1mm in size, light yellow and felt-like which does match the appearance of sclerotia in L. birnbaumii. Hennings compares them to Sclerotium mycetospora Nees ex Fr.[4]
2.1. Sclerotium mycetospora and S. sinapispermum
Nees covered Sclerotium species extensively in his own work[5] but the description of Sclerotium mycetospora comes via Fries in 1822. They are said to be like mustard seeds in habit, gregarious, starting pubescent and white. He notes they favour the light, grow amongst the bark in hothouses and grow into Agaricus volvaceus and so they are compared with Sclerotium pubescens which he says also grow into Agarics:[6]
S.? mycetospora, liberum, subtectum, globosum, lævo, stramineo-album, basi tenui byssinae insidens.
S. mycetospora. Fr. Nees in litt.
Habitus seminis Sinap. albæ, gregarium; primo obsolete pubescens, album; mox glabriusculum, stramineum. Observante Cel. inventore astate, favente luce! (h. e. non obtectum in Agaricum volvaceum excrescit. Inter corticem in Caldariis. (v. s.)
Cum status modo elementaris & e vegetatione, luce priva, personatus sit, strictissime omittendus; tamen ad indolem reliquorum, quibus simillimum, illustrandam maxime confert, quare inserere non dubitavi, Cf. Scl. pubescens, fungorum etc, quæ etiam Agaricos progignunt.
In 1863 Gérard Daniel Westendorp described Sclerotium sinapispermum as growing from tan bark in a hothouse in Menen, Belgium. They were noted as being spherical, smooth, 0.5-1mm in diameter and starting yellowish before maturing to orange and then red-brown with a distressed surface when dry and free from the mycelium:[7]
Scler. sinapispermum n. sp.
Péridium sphérique, d'un demi à un mill. de diamètre; à l'état frais d'abord jaunâtre puis orangé, lisse et adhérent par un point; à l'état sec libre, d'un rouge brun et légèrement chagriné à la surface. Chair cornée blanche.
Sur la tannée, dans une serre chaude à Menin, chez l'horticulteur Vander Plancken.
This description seems to strongly align with that of Leucocoprinus sclerotia in regards to the dimensions, colour and habitat. The note of them adhering to a point is also of great relevance as this trait becomes very apparent and at times quite frustrating when trying to study the sclerotia in various Leucocoprinus species. They will readily stick to a needle tip, scalpel or any instrument used to manipulate them. As such attempts to locate them on a slide or dissect them can prove frustrating although in practice it does mean that isolated sclerotium or small clusters can be purposefully collected for study simply with the tip of a needle.
In 1867 Jean Kickx suggested that S. sinapispermum was synonymous with S. mycetospora.[8] In 1889 Victor Fayod noted that Sclerotium mycetospora belonged to Volvaria volvacea (now considered a synonym of Volvariella volvacea).[9] This association however seems to only be based on the previous one with Agaricus volvaceus and Fayod does not provide any more information. Sclerotia were also noted by Adalbert Ricken in 1915 who cited Hennings description and attributed them to Lepiota cepaestipes, describing the cap of the mushroom as whitish, yellowish or even sulphur yellow.[10]
In 1918 Sclerotium mycetospora was categorised by Mattirolo as having white sclerotia and classified as Lepiota incerta.[3] In 1948 Karel Cejp reclassified this as Leucocoprinus flos-sulphuris var. incerta.[11]
The association with Volvariella volvacea makes the identity of the species Fries was describing unclear and the mention of Sclerotium pubescens also growing into an Agaric species is confusing. Christiaan Hendrik Persoon described Sclerotium pubescens in 1801 as gregarious, globose and pale with a hairy base and said they were found in Autumn on the gills of a decaying Agaric mushroom, the type of which is not further specified. This does not sound like a species which grows into an Agaric mushroom but rather a fungus that decomposes them. Little other detail is provided besides them being 1 line wide.[12] This would correspond to ~2mm or ~2.25mm so seems too large for the sclerotia of a Leucocoprinus species unless it is greatly rounded up. Collybia tuberosa and similar species grow on decomposing mushrooms and produce sclerotia which are around this size or larger so may provide an explanation.
Additional sources of confusion for Leucocoprinus sclerotia may be some Aspergillus and other mold species growing on decomposing mushrooms. It is not uncommon to see observations in which sclerotia of L. birnbaumii are found alongside Aspergillus or other molds which are either decomposing the mushrooms or consuming material in the plant pot. If both were found together it could easily cause confusion with the assumption that they were related due to their similar appearance. The sclerotia and associated mycelium do have an appearance similar to many molds.
 |
 |
|---|---|
| Observation 142511321- Aspergillus growing on a mushroom Credit: @pipsissewa | Observation 142511321- Aspergillus close up Credit: @pipsissewa |
 |
 |
|---|---|
| Observation 150294254- Hypomyces species on polypore Credit: @komille277 | Observation 3922092- Moldy Leucocoprinus Credit: @williamgarner |
2.2. Sclerotium hirsuta and Periola hirsuta
Periola hirsuta was Elias Magnus Fries' 1822 reclassification[13] of Sclerotium hirsuta as described by Heinrich Christian Friedrich Schumacher in 1803.[14] As Mattirolo points out however there are numerous issues with the reclassification and description as Periola. This description is provided by Fries:[13]
P. hirsuta, obconica, hirsuta, ochroleuca.
Scl. hirsutum. Sehum. Saell. 2. p. 187. Fl.Dan. t.1320,
Sparsa, simplex, superficialis, 2 lin. fere longa, subturbinata, intus pallida, versus basin umbrina. Substantia carnoso-gelatinosa. Ad Rhizomorpham subcorticalem supra truncos vetustos fagineos. Sept. (v. ic.)
The habit described here misses some critical features of the original description and seems to suggest growth on old Beech trunks. Fries also cites Florae Danicae Iconum tab 1320 (MCCCXX).[15] This illustration appears to be something unrelated. Based on the illustration and the description provided by Fries it would seem doubtful that this is a description of Leucocoprinus sclerotia at all. However the original description by Schumacher[14] provides more detail:
S. hirsutum, obconicum subturbinatum, hirsutum, ochroleucum, intus pallidum basin versus umbrinum; substantia carnoso-gelatinosa.
In vasis exsiccatis subcorneis trunci cæfi Fagi sylvaticæ. Septembr.
It specifically notes that they were growing in vessels containing the dried Beech bark - a common habitat in which to find Leucocoprinus species with many of the early descriptions being from bark beds in hothouses. Given this context the more appropriate translation of vasis may be vases or plant pots but no further information is provided.
Mattirolo addresses these issues and states that the illustration represents greatly enlarged structures and that it did not correspond to the truth, the specimens having been communicated to the artist by Schumacher rather than observed and drawn by the same person. He notes that the size given by Fries of 2 lines long corresponds to 4.5mm, stating that this is about five times larger than the actual size and that Fries arrived upon this size by measuring the fungi depicted in the illustration rather than observing specimens personally.
Mattirolo notes the importance of the colour description given and provides a definition[3] (translated from Italian via Google):
Ochroleucus, means exactly one color, albo-flavidus, corresponding to that of candle wax; but not to that of beeswax. This name derives from ochros == yellow earth, and leucos == white. Since it is important for our study to fix exactly the value of the Chromotaxia colors of P. A. Saccardo. Editio alter. Patavii, 1894.
Kew's website notes that confusion can arise over colour citations given in Chromotaxia due to the alteration of the colours in the book with age.[16] So this definition, which is just buried amongst the references in the footnotes on the page is more useful than it may appear as the surface of the sclerotia can indeed appear wax like with some variation in colour between species.
Mattirolo goes on to cover, in great detail the string of errors surrounding P. hirsuta that resulted in another seemingly erroneous illustration being created by August Karl Joseph Corda in 1837-38 in Icones Fungorum. A citation of volume II, tab XIII, fig. 160 is given however this appears to be a transposition error as the illustration is actually figure 106.[17]
Corda describes the entire outer surface as being covered in flakes and polygonal spores arranged one after the other like beads on a thread and says that these spores were able to germinate and produce new fruiting structures.[17] In 1910 Teodoro Ferraris also illustrated and described P. hirsuta with conidia and stated that it was affiliated with Volutella.[18]
Mattirolo concludes, based on his own contamination prone attempts at cultivation of the sclerotia, that the spore bearing conidia described by these authors as being present on the surface of P. hirsuta are either the result of mold such as Penicillium growing on the sclerotia or the internal tissue of the sclerotia spilling out when crushed. Based on my own observations however I would posit another theory - that these authors may not have even been observing the sclerotia.
 |
 |
 |
|---|---|---|
| Fig. 1 - Chaetomium contaminant found in L. cretaceus culture. | Fig. 2 - Corda's illustrations including Periola hirsuta | Fig. 3 - L. cretaceus sclerotia from agar erupting with crystals. |
The Chaetomium species (fig. 1) that has contaminated many of my own attempts to culture Leucocoprinus species would appear to resemble Corda's illustration of Periola hirsuta (bottom left corner of fig. 2). In some cultures this was observed to be growing side by side with the sclerotia and the immature forms of them were initially indistinguishable to the naked eye, appearing only as tiny white specks scattered gregariously across the substrate. As the Chaetomium developed further it became greenish yellow and ultimately dominated in most of these cultures eventually colonising the entire jar and appearing like a greenish black or grey mold throughout. This appearance is not wholly dissimilar to a jar colonised by L. cretaceus in which the vast number of mature, black sclerotia amongst the white mycelium results in an overall grey, mold like look.
In others where Leucocoprinus sclerotia were noted and aborted primordia were present, spores resembling those of Chaetomium were also found amongst the crushed sclerotia when examined under the microscope. This initially caused confusion in identifying the sclerotia as whilst the number of spores was comparatively few it could appear as if they were spilling out of the crushed sclerotia. Chaetomium spores are somewhat similar in appearance to those of L. cretaceus so it was not clear if the spores were left over from inoculation or if they belonged to the Chaetomium species. Attempts to culture these sclerotia however instead produced jars filled with Chaetomium. They are immediately distinguishable from sclerotia when examined microscopically due to the vast numbers of spores that spill across the slide when crushed - a feat that was easy with the Chaetomium, which offered little resistance compared to the sclerotia that took force to crush.
Additional confusion was caused by the crystals that spilled out of some crushed sclerotia that were cultured on agar (fig. 3), at low levels of magnification these looked as if they could have been spores and were quite polygonal in form, as many crystals are. At higher levels of magnification it became abundantly clear that they were crystals and they were also noted in agar around the tips of the hyphae that were forming into sclerotia. This potentially suggests that this aggregation of crystals represented the crystals collected from many hyphae as a store of nutrients in the centre of the sclerotia however whether this phenomenon is just the product of growing in an agar mix with a high sugar content has not been evaluated yet. Distilled water was not used for this agar mix and the water has a high mineral content so Calcium oxalate crystals are also a possibility. Some crystals appeared colourless whilst others had a brownish yellow colour, similar to that of the malt extract agar itself so there may have been multiple different crystalline structures present. Some crystals have also been noted in the sclerotia of L. cretaceus and L. cepistipes cultured on water agar but such crystalline structures have so far not been observed in sclerotia collected from other substrates so may simply be from the agar.
With or without crystals, the sclerotia and the Chaetomium could reasonably be conflated and confused and it may be that they are only easily distinguished with a modern microscope and camera to compare images. This common soil fungus which appears somewhat similar to the sclerotia of Leucocoprinus species may explain the confusion in this illustration and Corda's description. When the outer casing of the sclerotia turn black with age they appear quite similar to the black centre of the Chaetomium fruiting bodies and the yellowish green hairs on them could appear like some other mold. Additionally, as neither Corda or Ferraris remark on the hardness of the structures or note any difficulty in crushing them beneath a cover slip it does not seem likely that they were actually examining Leucocoprinus sclerotia. The hardness is such that mounting them for examination is not trivial and so it seems like it would be impossible not to make note of this trait.
Interestingly it was Corda who gave L. birnbaumii the specific epithet that it has today in the third volume of Icones Fungorum, it famously being named for a Mr Birnbaum, a gardener in Prague who found the species growing amongst pineapples in hothouses in the Salmovský gardens. Yet in his description of L. birnbaumii Corda does not note sclerotia.[19]
Other mycologists of the time seem to provide vastly different descriptions of P. hirsuta that more closely fit those of the sclerotia of a Leucocoprinus species. In 1899 Domenico Saccardo provided a description of P. hirsuta from sphagnum moss in plant pots in a greenhouse in the Treves garden in Padua, Italy:[20]
Sporodochi superficiali, rotondi, internamente duretti o carnoso-gelatinosi. Conidi globoso-ovoidei, tipicamente disposti a catenella, fra le setole, continui, ialini.
— Sporodochi sparsi, quasi rotondi, un po' pelosetti, 0,5-0,7 mm. diam., esternamente bianchi ed all'interno duretti e di color nocciuola pallido; ife del contesto irregolarissime, intrecciate, ramose, 6-10 μ. di grossezza, le periferiche ialine, filiformi, un po' ramose, grosse 3 μ., spesso granulose. Conidi globoso-cubici, ialini. Sulle foglie degli Sphagni sparsi sui vasi, in una serra del giardino Treves a Padova.
Whilst this description still mentions conidia the description otherwise sounds like a good match for the sclerotia of a Leucocoprinus species. The size of 0.5-0.7mm, almost round and hairy structure and the external white colour with a pale hazelnut colour internally are consistent if examining sclerotia that are still encased in their mycelial envelope. As is the description of them as hard or fleshy and gelatinous since this can change with maturity. Dried material from this collection is stored in a herbarium so could be further evaluated.
Mattirolo states that he communicated details on this species in 1900 to P. A. Saccardo having made a collection from the sphagnum moss used as a substrate in orchid greenhouses in Florence and Turin. This was followed by a further collection by D. Saccardo in 1904 from orchid greenhouses in Rome where it was described as growing easily even on the bare backs of vases and not being harmful to the orchids or other moss covered plants. This is describing a typical trait of L. birnbaumii sclerotia which is commonly seen in observations on iNaturalist and houseplant forums. They have a tendency to grow on the underside and around the base of terracotta pots and in the drip trays, often in vast numbers. The association with orchids has also been frequently noted with professionals in the modern Orchid industry regarding them as problematic due to their appearance reducing the decorative value of the plants.[21]
In 1918 Sclerotium hirsuta was categorised by Mattirolo as being the species with the ochroleucus sclerotia and was classified as Lepiota flos sulphuris,[3] a reclassification of Schnizlein's Agaricus flos sulphuris.[22] In 1948 Karel Cejp reclassified it as Leucocoprinus flos-sulphuris.[11]
2.3. Lepiota flos-sulphuris and Lepiota lutea (Mattir.)
In 1851 Adalbert Schnizlein described Agaricus flos sulphuris growing from moss used to top the beds in a hot house in the botanical garden. He says it often appeared after the moss had been in use for one and a half years and whilst he does not explicitly describe sclerotia, he does note that the mushrooms arise in many places simultaneously in great numbers in masses agglomerated by mold, which he described as a very pretty sight. This is accompanied by an illustration of Agaricus (Lepiota) cepaestipes Sowerby, but it is completely yellow, again suggesting that it was L. birnbaumii which was observed.[22] Schnizlein cites Ludwig Rabenhorst's description of Agaricus cepaestipes that is similar to his observation but white[23] and so the name Agaricus flos sulphuris meaning flower of sulphur was used instead because of the yellow colouration.[22]
Mattirolo acknowledged that William Withering's Agaricus luteus, Corda's 'strangely named' Agaricus birnbaumii and others may have been analogous to the yellow species he was observing however he settled upon Lepiota flos-sulphuris to distinguish it from Lepiota lutea which he also described. Lepiota flos-sulphuris was described as scaly, flocculose and bright sulphur yellow with concolourous gills and orange flecks in the centre of the cap. A spore size of 8-10 x 4-6 μm is given. Mattirolo only noted this species growing in greenhouses with tropical plants so concluded it was a species of tropical origin that was introduced to Europe with these plants, though noted it could spread to others and was not dependent on them.[3]
Mattirolo observed ochroleucus sclerotia associated with this species which had a creamy coloured mycelial envelope that was 10 µm thick on average whilst the overall sclerotia reached just 1mm diameter on average. The mycelial envelope hardly disappeared when rolled between the fingers and a repeated force was required to remove it, resulting in yellowish brown colouration showing through and the sclerotia becoming smaller. When fresh they resist crushing and 'escape the razor cut' and when dried they have the hardness of grains of sand. They retain their colour in alcohol with the mycelial coating not changing in character or colour. The sclerotia grow very close to each other with a yellowish bysoid mycelium holding them together.[3]
Lepiota lutea was described as very close in its general appearance but with the sulphurous yellow colour of the mushroom being lighter and lacking the orange colouration. It has a slightly swollen but not bulbous stipe which is scaly or hairy but lacks the flocculose coating. More importantly the spore size is far smaller at 4-5 x 3-4 μm and so it is clearly distinct. Mattirolo noted this species as growing on terra di castagno† and on old trunks of various species. It was not exclusive to the greenhouse environment with it being found outside in the regions of Piedmont and Lombardy, Italy always on terra di castagno. From this he concluded that this species was not of tropical origin and was native to Europe. Sclerotia were not observed in this species.[3]
† Terra di castagno is a substrate of decomposing chestnut wood which literally translates as 'chestnut earth' or 'chestnut soil', though does not yield results in English for these search terms. Italian results describe it as very acidic, nutritious and excellent for almost all plants.
Recent ITS sequences posted to iNaturalist with accompanying photos do suggest multiple species going under the name of L. birnbaumii and genbank results are fairly inconsistent with many sequences submitted under this name that are only 90-95% matches with others. It appears that there are in fact a number of similar yellow Leucocoprinus species found in plant pots which routinely get confused. Observation 135980665 by @sarahmycena and 26909898 by @pycnoporus with sequences provided by @stevilkinevil appear to show paler specimens than is seen with 126103447 by @kev317. The sequences for these are distinct as is observation 19645434 by @alan_rockefeller from a pile of wood chips in California which likewise differs genetically and appears to have a slightly different colouration. Appearances can vary however due to rain or watering washing out the yellow pigment in some specimens resulting in a pale looking cap with brighter yellow centre and so distinguishing specimens via macroscopic characteristics is complicated by environmental conditions.
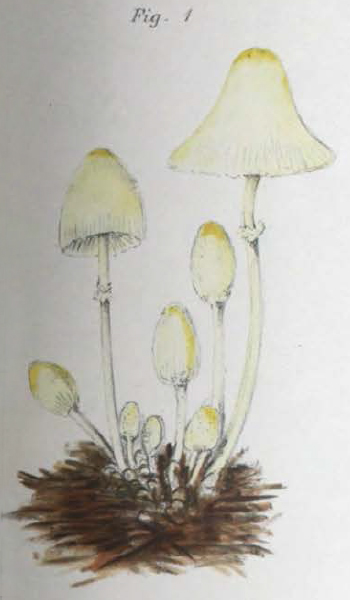
This does not appear to be the case with Mattirolo's Lepiota lutea however as it has a spore size more consistent with that of Leucocoprinus straminellus or L. medioflavus. The photos Mattirolo includes are in black and white but do look similar to the few confirmed photos that are available online or in papers for these species and his illustration (fig. 4) also confers with them, showing pale mushrooms with a brighter yellow centre. By contrast Mattirolo's illustration of Lepiota flos-sulphuris (fig. 5) looks like L. birnbaumii.
Differentiating Leucocoprinus straminellus, L. flavescens and L. medioflavus in photos is as yet unclear to me especially given the virtually identical white variants Leucocoprinus straminellus var. albus and L. medioflavus var. niveus.[24] Observations for these species are not as common as for L. birnbaumii but are by no means rare either so in time it should be possible to acquire enough samples to try and distinguish them. For the time being I have just been collecting observations of them here in order to better compare them. Many of these observations appear to match what Mattirolo was observing with Lepiota lutea. I have so far only examined the spores of one such specimen with a similar macroscopic appearance and a small spore size that fell within this range. Attempts to culture it however resulted in very slow growth that ultimately succumbed to contamination with efforts to save it proving futile vs the contaminants that grew far quicker. A culture was left to grow on wheat bran for some months however and in this period no sclerotia were observed though further study would be needed.
iNaturalist observation 134794364 by @pogona_vitticeps seems to show a pale species with a brighter yellow centre developing abundant sclerotia in a bag of potting soil however the mushrooms are under developed or possibly deformed and so it is not clear what they are. A similar appearance is found in observation 173147298 and 174912287 by @jarenjaren and this one has recently been sequenced with the results showing only a 92-93% match with L. birnbaumii and a 96% match with L. straminellus. So L. medioflavus might be the correct identity for this observation but microscopy will be required to confirm. Observation 183108232 by @hikasukepon also appears similar and has microscopy which also suggests L. medioflavus as a probable ID.
 |
 |
 |
|---|---|---|
| Fig. 4 - Lepiota lutea | Fig. 5 - Lepiota flos-sulphuris | Fig. 6- Lepiota incerta with sclerotia |
2.4. Lepiota incerta
Mattirolo described Lepiota incerta as a new species so no synonyms are listed. The cap and gills are described as straw-white (stramineo-albo) with a stem that is flocculose with a bulbous white base and Isabelline (pale cream brown) around the annulus. The spore size is given as 8-10 x 4-6 μm, the same as for Lepiota flos-sulphuris but also similar to many species of Leucocoprinus that have been described by others. He describes minute scales in the centre of the cap but when he says 'ad centrum tantum carnosulo' 'only fleshy in the centre' it is unclear if this is in reference to the thickness of the flesh or a fleshy colour, since his illustration (fig. 6) does show a brownish or creamy brown colour around the centre that is not otherwise noted in the description.[3]
With this species, Mattirolo observed white sclerotia with a diameter greater than 1mm with a tomentose, milky white mycelial envelope that was 3-4 µm thick. It disappeared easily when handled between the fingers resulting in the sclerotia becoming much smaller and a hazelnut colour showing through. They had a more gelatinous consistency making them more easily crushed and dissected and when dried they were not as hard as the ochroleucus ones. In alcohol they immediately lost the white colour with the mycelial envelope becoming transparent and a distinct brown colour showing through. The sclerotia grew spaced apart and linked together by white mycelial cords but lacked the bysoid mycelium.[3]
Mattirolo compared the mushrooms with Lepiota cristata, Lepiota tenella (=Leucocoprinus tenellus), Lepiota serena (=Leucoagaricus serenus), Lepiota morieri (=Cystolepiota seminuda) and Lepiota brebissoni (=Leucocoprinus brebissonii). The greatest affinity was noted as being with Agaricus (Lepiota) straminellus (=Leucocoprinus straminellus), the dried specimens of which are said to be so similar as to appear identical.[3]
It is unclear to me which species Mattirolo was observing but based on the illustration I believe the best contender could be Leucocoprinus ianthinus given that it is also commonly observed in plant pots. However based on some differences apparent in the macroscopic features and different microscopic features noted by many authors it seems likely that there may be two similar species so some of these observations could be L. lilacinogranulosus. As this species is currently considered a synonym by Species Fungorum and Mycobank observations of L. ianthinus may represent a collection of both species.
iNaturalist observations would suggest that Leucocoprinus brebissonii is commonly found in the wild worldwide with many observations especially on the West coast of the United States but it does not seem to be a common find in plant pots. A review of the over 700 observations of this species on iNaturalist (research grade, casual and 'needs ID') has not turned up any definite observations of this species in plant pots.
By contrast the over 300 observations of L. ianthinus on iNaturalist suggest they are almost exclusively found in plant pots. There are some observations from the wild in Florida, Georgia, Alabama and Louisiana which commonly get suggested as L. ianthinus by the iNaturalist algorithm and indeed do look quite similar, though it is currently unclear which species they are. There are also similar observations from Australia, though these generally have some slight differences in appearance to the American ones and it is possible that L. austrofragilis may match some of the Australian ones.
It is not clear where L. ianthinus is actually native to or how it became introduced into Europe and the rest of the world. Exploring the taxonomy on some of the common houseplants of tropical origin like Pothos (Epipremnum aureum), Montserra and Dieffenbachia with which these mushrooms are commonly found could suggest possible origins, especially in cases where the plants were introduced to hothouses in Europe shortly before the mushrooms were described (Pothos and Agaricus ianthinus were both described in the 1880s). However as the description of L. ianthinus does not give any information on the nearby plants in the hothouse at Kew in which it was found and the text on Pothos does not explicitly mention when or if specimens were sent to Kew this is at most speculative and inconclusive.
Flora Agaricina Neerlandica gives a spore size for Leucocoprinus brebissonii of 8.5-13 x 5-8 μm or 9.3-11.5 x 5.6-7 μm on average and for L. ianthinus it notes 8-12 x 5.5-7.5 μm or 9.4-10.4 x 6.5-6.7 μm on average.[25] Mattirolo's Lepiota incerta is close at 8-10 x 4-6 μm but would not seem to be a strong match to the range given here. Derek A. Reid gives a smaller spore size for L. ianthinus of 6.5-10 x 5.75-6.5 μm in contrast to a size for L. lilacinogranulosus of 7.5-9.75 x 5-7 μm or 6-7 x 4.75-6 μm. L. lilacinogranulosus var. subglobisporus is noted as having globose or subglobose spores at 4.5-6 x 4.2-5 μm.[26] Jean Louis Émile Boudier produced illustrations of Leucocoprinus tenellus which appear similar to Mattirolo's illustration of Lepiota incerta[27] however a spore size of 12-14 x 7-8 μm is given.[28] Mattirolo noted this species as being related but differing in colour, type of annulus and spore size.
Other similar looking species have been described such as Leucocoprinus otsuensis from Japan in 1953 by Tsuguo Hongo which was said to be closely related to L. brebissonii but being easily distinguished by the scales on the cap, which are dark brown. The spore size given is 9-11 x 6.5-7.5 μm or 10-13 x 6-8 μm.[29] Species Fungorum lists the current name as Lepiota otsuensis Hongo whilst Mycobank has it as a synonym of L. brebissonii.
The uncertainty of which species Mattirolo was dealing with is reflected in the name incerta which is Latin for uncertain, a name which Mattirolo said he gave the species as it signified his thoughts. It is easy to see why Karel Cejp considered this species to be a variant of Leucocoprinus flos-sulphuris given the identical spore size, the presence of similar sclerotia and Mattirolo's uncertainty as to its identity.
It could even be tempting, were Mattirolo's work less detailed and thorough, to dismiss them as just the same species. The illustration of Lepiota incerta has a colouration quite similar to that of many Leucocoprinus species when they are old or dried where the yellow colours become more grey brown or beige. The larger, white sclerotia that are gelatinous and less hard could represent a more immature form which appears larger before the softer tissue hardens and loses water content. Collecting hundreds of observations of Leucocoprinus sclerotia in an iNaturalist project has made it possible to easily compare them and look for differences and similarities. Colours appear quite variable from a distinctly yellow to white or more of a beige colour but bright yellow mushrooms that appear to be L. birnbaumii are present in enough of these observations to suggest that these sclerotia belong to the same species regardless of colour, hence distinguishing the sclerotia by colour may not be reliable. Sclerotia in L. cretaceus and L. cepistipes also appear white when immature and it seems likely that if other Leucocoprinus species produce sclerotia they will be similarly white like the mycelium.
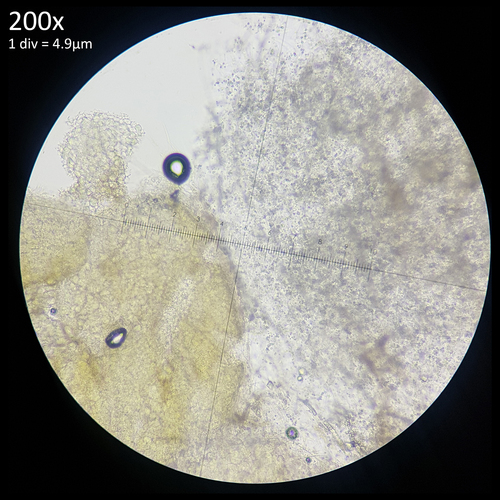
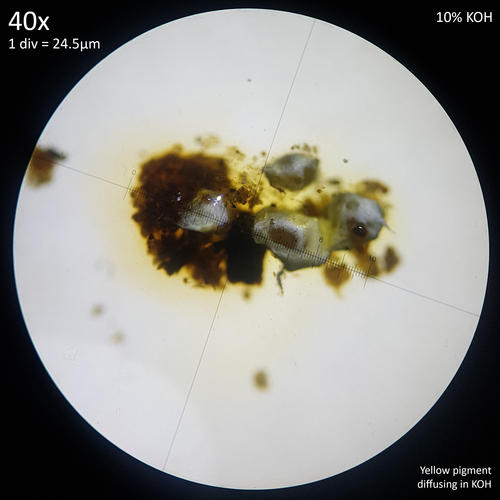
Additionally the yellow pigment present in the sclerotia or mycelium of L. birnbaumii leaches out and diffuses into a solution of 10% Potassium hydroxide (fig. 7). This may be Birnbaumin A or B, the same 1-Hydroxyindole pigments that have been isolated from the fruiting bodies via methanol extraction.[30] Considering the solubility of this pigment it may be possible that when tap water is used to water potted plants the Sodium hydroxide that it can contain results in sclerotia that lose the yellow colouration and appear white. Possibly reactions could also occur with naturally occurring hydroxide ions produced in water as a result of an ionisation reaction or with other substances either added to drinking water, such as chloramine or chlorine or found naturally in it such as various dissolved minerals.
Mattirolo notes that the sclerotia of Lepiota flos-sulphuris and of Lepiota incerta were confused by previous authors due to both living on the same substrate in greenhouses. However his own study was also conducted in this environment with attempts to culture either the sclerotia or mushrooms being unreliable and failing due to contamination. Therefore the bulk of his observations were made on specimens growing in the greenhouse and the associated sclerotia were identified by their proximity or attachment to the mushrooms rather than through observation in isolated cultures.
 |
 |
|---|---|
| Fig. 7 - Yellow pigment of L. birnbaumii sclerotia diffusing in KOH | Observation 176917949- L. birnbaumii and L. ianthinus Credit: @tx_tea |
Since Mattirolo's work is comprehensive and was conducted over many years with sclerotia illustrated at the base of the mushrooms and as the appearance of dried Lepiota incerta is compared to other herbarium specimens, it seems safe to rule out these species simply being the same.
It is however worth noting that he was never able to successfully culture the sclerotia to produce mushrooms and every attempt to grow them, by his own admission, succumbed to contamination and failed. He was able to demonstrate that no conidia were present on the sclerotia, as other authors had claimed, by observing their growth for years in the greenhouses of Turin and Florence. He was regrettably not able to further study their growth in isolation however due to cultures failing. He describes his attempts at cultivation as fruitless despite trying many times over the years.
The main issue that plagued Mattirolo's efforts to culture these species appears to be a combination of contamination and perhaps less than ideal substrates. He tried sphagnum moss, grapes, plums, terra di castagno and 'various jellies' or gelatines though does not mention what processes were used to sterilise these substrates or to provide a sterile growing environment. He describes sclerotia and cultures readily falling prey to mold, often after the appearance of colourless 'brilliant' water droplets/exudation which turned yellow and opaque followed by mold appearing.
In a culture of Leucocoprinus cretaceus grown on malt extract agar I observed similar exudation appearing shortly after the development of the sclerotia, which were produced in abundance compared to the water agar formula that had little growth. The droplets were at first crystal clear and gave the top of the substrate a brilliant, glistening appearance like early morning dew on grass. They persisted for several days and grew to a similar size as the sclerotia themselves before some yellow orange discolouration occurred within the liquid. These droplets ultimately disappeared, either through evaporation or reabsorption leaving the sclerotia looking dry, however no contamination occurred within this culture. The exudation appears to be related to the development of the sclerotia and the result of losing water as they mature, harden and dry. This has only been noted on agar however and not observed in jars of rice, wheat bran or wood so it is unclear if it is affected by the saturated substrate providing excessive water. Such exudation is commonly remarked upon in studies of sclerotia in many different species however and appears to be a common trait.
Mattriolo's experimentation appears limited by the tools, materials and knowledge of the era. He notes that attempts to cultivate cultures from isolated sclerotia only resulted in contamination whilst cultures from myceliated substrate were able to produce new mycelium and hyphal knots that formed into small sclerotia, before they too fell prey to contamination. The sclerotia production never reached that which happened naturally in pots in greenhouses even when attempts were made to control the temperature.
It may be for this reason that he stated that Lepiota cretacea (now known as Leucocoprinus cretaceus) did not produce sclerotia since they are not easily observed in specimens growing naturally in soil but are readily apparent in cultures, especially in high nutrient substrates.
In his observations of the species that he described as Lepiota flos-sulphuris, Mattriolo notes that the sclerotia and mushrooms are only observed on the Sphagnum moss lining the tops of pots after they have been in the pot for at least one year. He states that if the moss is removed and replaced with new moss it takes another year before sclerotia or mushrooms reappear. He also notes similar with the growth in terra di castagno when used to cultivate tropical plants.
These observations match those we see today with people regularly noting mushrooms or sclerotia only appearing in their plant pots after they've already had the plant for several years, which adds to their surprise and confusion when they find them. It is also important to note that Mattriolo was writing from a time before the modern potting soil mixes we use today were formulated. Commercially available potting mixes comprised of peat moss wouldn't arrive until the 1960s and it is only more recently that coco coir has come to replace this out of environmental concerns. Earlier observations of Leucocoprinus species almost always note bark beds as these were commonly used in hothouses however Sphagnum, terra di castagno and modern potting soil mixes may provide a more ample habitat that encourages greater production of sclerotia. These substrates may provide a similar environment to the light, airy and moist topsoil of rainforest floors and are likewise rich in undigested organic material. This could explain why the sclerotia of Leucocoprinus birnbaumii are observed so readily today yet were not so commonly remarked upon in earlier observations.
2.5. Cenococcum xylophilum & Leucoagaricus meleagris
There are some additional descriptions which resemble the sclerotia of L. birnbaumii which were not covered by Mattirolo. In 1829 Fries described the Cenococcum genus as containing two species with C. geophilum citing Sowerby's Lycoperdon graniforme as a synonym and C. xylophilum as a new species. Cenococcum are described as small black balls about the size of vetch seeds which are not rare but are easily overlooked amongst black soil. At first the interior is similar to the exterior but then becomes filled with powdery spores and leaves a hollow in the centre with brown spores noted in C. geophilum and white spores in C. xylophilum. However no spore measurements or descriptions are otherwise given.[31]
Lycoperdon graniforme was found in woodland in England where it appeared like shot pellets lying on top of the ground. They were brittle and cracked easily revealing a black powder and are illustrated as little more than rough black balls containing this powder.[32] This can surely be disregarded as Leucocoprinus sclerotia however as the description of Cenococcum xylophilum is heavily based on direct comparison with C. geophilum it was necessary to explore this.
C. geophilum was found in England amongst beech woodland or dried up basins in Autumn and a variant is also noted from France. C. xylophilum is described as having a pale purplish floccose thallus with mature peridia that are only loosely attached. The outside is said to be similar to sclerotium but the inside is floury whitish. It was found in woodland in Petropoli, Russia.[31]
The sclerotia of C. geophilum are similar in appearance to the sclerotia of Leucocoprinus cretaceus appearing as they do as small black balls. However as it is an Ascomycete fungus that is ectomycorrhizal this is clearly unrelated.[33]
In 1900 Jean Louis Émile Boudier and Narcisse Théophile Patouillard reclassified Cenococcum xylophilum as Coccobotrys xylophilus. It was noted as being a true sclerotium and described as having minute 1-2mm wide, hard irregularly round or pear shaped structures with an ochreous-yellow exterior with a black surface beneath followed by a reddish layer under that and a paler centre. This centre area is more friable with a pale ochreous colour that often turns red or whitish when it dries out. They were found during April in bark beds in a hothouse growing palms. These numerous growths arise from rhizomorphic mycelium that is ochreous-fawn in colour. The outer layer of the sclerotium is said to be exfoliated by boiling lactic acid and not coloured by the addition of a hot solution of cotton blue added to the acid. The interior of the cells is filled with granular protoplasm which starts white before becoming ochraceous. The protoplasm is stained with iodine in the same manner as glycogen/starch. The granular structure simulates the appearance of spores though Boudier and Patouillard do not describe finding any spores and since no mushrooms are described it does not appear that fruiting bodies were observed and associated with the sclerotia.[34]
The colour, hardness and habitat described here as well as the black and ochreous-yellow layers does sound like the sclerotia of L. birnbaumii. However the size given is much larger and it is unclear what the repeated mentions of a red colour refer to. The French text only says 'rouge', several times and the Latin uses 'rufis' so it is not clear what particular shade is being described. Reddish brown could be an adequate description of the orangy brown colour seen prior to the black colour developing in the sclerotia of L. cretaceus and when L. cepistipes is cultured on agar a reddish brown colour is sometimes present in the old mycelium surrounding the sclerotium, with similar noted in descriptions of some Coprinopsis species however this colour is more brown than red.
Coccobotrys xylophilus is described as being very reminiscent to the Emericella genus with a citation that points to Patouillard's Emericella variecolor (now known as Aspergillus stellatus). The similarity that is referenced appears to be the presence of the hairy, yellowish, globose or pear shaped tubers which are 1-3mm high and scattered or grouped on rotten wood.[35] However it is noted that C. xylophilum is very different from this species by virtue of the sclerotia.
In 1900 Charles van Bambeke described the 'very rare and hardly known spherical grained mycelium' of Coccobotrys xylophilus as an asexual morph belonging to Lepiota meleagris (now known as Leucoagaricus meleagris). The specimens studied by Bambeke were found growing on the tan bark in a hothouse in Belgium. Little further detail is added to describe them.[36]
However it is noted by Else Vellinga that the material examined by Boudier and Patoulliard and Bambeke was not the same as the Cenococcum xylophilum examined by Fries.[37] This is indeed very evident by the differences in the descriptions given and as such it is unclear which species were being described by these authors, suffice to say that some of them do resemble the sclerotia of a Leucocoprinus species.
2.6. Additional studies
In 1948 Karel Cejp reclassified Mattirolo's Lepiota flos sulphuris as Leucocoprinus flos-sulphuris and Lepiota incerta as Leucocoprinus flos-sulphuris var. incerta. Cejp personally observed L. flos-sulphuris growing in greenhouses as well as L. cretaceus, L. cepistipes and L. meleagris. Little further information is provided on the sclerotia of L. flos-sulphuris however and sclerotia were not observed in any of the other species.
Cejp states that L. flos-sulphuris can be easily confused with the similar yellow species described from greenhouses Leucocoprinus luteus (With.) Pat. and Lepiota lutea (With.) Godfrin. He notes that the authors of them were probably not familiar with the sclerotia always created by L. flos-sulphuris but goes on to state that Agaricus cepaestipes and Agaricus cretaceus did not produce sclerotia.[11]
This speaks to the distinctive nature of sclerotia in Leucocoprinus birnbaumii such that they are far more easily observed than those of L. cepistipes or L. cretaceus which would be easily missed without culturing these species.
Cejp states that Corda's Agaricus birnbaumii is probably identical to L. flos-sulphuris. His reclassification of Lepiota incerta does not appear to have been based on personal observation however and no further information was provided on this species with Cejp stating that it differs only in the colour of the mushrooms. Cejp also says that Leucocoprinus flos sulphuris var. nigrescens-minor is a variety which is just a coloured form with mainly black coloured scales on the cap. The citation given points to Agaricus cepaestipes var. nigrescens Baglietto[38] but there does not appear to be any information in this description to assume an association with L. flos-sulphuris. There does appear to be a variant or species similar to L. cepistipes but with a darker centre to the cap which is seen occasionally in observations so this is likely what Baglietto was describing.
A study in 1962 was successful in culturing sclerotia of Leucocoprinus luteus (now considered a synonym of L. birnbaumii) on wheat chaff until they fruited. Warcup and Talbot state that they were unable to determine whether sclerotia had previously been noted in this species. However they cite L. cepaestipes as a similar species that possesses pale or light yellow sclerotia so this may just be another example of the taxonomy being confused with these species.[39]
In 2011 a study on sclerotia of L. birnbaumii found in a plant pot in Japan confirmed via genetic sequencing that the sclerotia were the same species as the mushrooms.[2] This study does not cite any of the sources used here that reference sclerotia or any of the early descriptions of the Sclerotium species so it seems that the authors were unaware of them. This would not be surprising as this study appears to be the only modern one which discusses sclerotia in a Leucocoprinus species in any detail.
3. Sclerotia in Agaricales
The 2014 study 'How many fungi make sclerotia?' provides an excellent place to start researching sclerotia with many sources collated. When it comes to Agaricales the list of species is short and incomplete but it does provide some genera to research further. The study lists the following saprotrophic Agaricales as producing sclerotia: Leucocoprinus luteus, Pleurotus tuber-regium, Coprinus lagopus, Coprinopsis sclerotiorum, Agrocybe arvalis, Psilocybe caerulescens, Stropharia tuberosa, Collybia tuberosa, Omphalia lapidescens and an unnamed Rimbachia species with a citation to the same Warcup and Talbot study that documented sclerotia in Leucocoprinus luteus, however I've been unable to find a description of it in that study. Three ectomycorrhizal species are also listed: Cortinarius calochrous, Hebeloma sacchariolens and an undescribed Entoloma species first documented in 'How many fungi make sclerotia?' Additionally one plant pathogen, Typhula incarnata is listed from the Agaricales.[1]
Many of these species, or their close relatives are common to find in potted plants. If it is to be considered that the sclerotia of L. birnbaumii may facilitate distribution of this species via potting soil, compost and potted plants then it is also necessary to explore the growth habits of other sclerotia producing species to evaluate whether sclerotia production appears to correlate with growth in potted plants in other species. Additionally such an investigation can reveal whether species in any other genera produce sclerotia similar to those of L. birnbaumii.
The iNaturalist potted plant mushrooms project has been collated manually via a mix of keyword searches, manual review and casual browsing. It is not a complete list of species found in plant pots and for many reasons does not and cannot provide a perfectly accurate representation of which of these species are the most common. For instance whilst Leucocoprinus birnbaumii is almost certainly one of the most common mushrooms to find in plant pots it is also one of the most spectacular, eye-catching and distinctive. People may be more likely to upload photos of this bright yellow mushroom that shows up and surprises them whereas the drab mushrooms produced by many species in the Psathyrellaceae family may not warrant so much attention, especially not after becoming used to them routinely showing up with seedlings every year. The project does however provide a useful reference for which species are typical to find in plant pots and which are not and enables easy comparison of these observations.
It would be remiss of any discussion on sclerotia not to mention H. J. Willetts comprehensive 1971 study 'The survival of fungal sclerotia under adverse environmental conditions'. This study covers commonalities observed amongst sclerotia such as the presence of melanin in the rind, the exudation of droplets observed during maturation as well as the potential resilience to contamination and extremes of heat and cold.[40] As such it is an invaluable tool in studying sclerotia despite it primarily being focused on plant pathogens, which are beyond the scope of this review. Coprinus stercorarius is briefly mentioned however. Other taxa that are discussed by Willetts with citations to studies on their sclerotia are:
Alternaria solani, Aspergillus, Aspergillus alliaceus, Aspergillus alliaceus, Aspergillus nidulans, Aspergillus phoenicis, Botrytis allii, Botrytis cinerea, Botrytis convoluta, Botrytis fabae, Ciborinia, Claviceps, Claviceps purpurea, Colletotrichum coccodes, Cordyceps, Fusarium solani, Helminthosporium sativum, Macrophomina phaseoli, Mucor hiemalis, Mycosphaerella ligulicola, Neurospora crassa, Papulospura, Phymatotrichum omnivorum, Pyronema domesticum, Rhizoctonia solani, Sclerotinia, Sclerotinia fructicola, Sclerotinia fructigena, Sclerotinia gladioli, Sclerotinia laxa, Sclerotinia laxa f. mali, Sclerotinia libertiana, Sclerotinia sclerotiorum, Sclerotinia trifoliorum, Sclerotinia (Monilinia) spp., Sclerotium cepivorum, Sclerotium rolfsii, Typhula, Typhula gyrans, Typhula intermedia, Typhula sclerotioides, Verticillium, Verticillium albo-atrum, Verticillium dahliae.
3.1. Sclerotia in Psathyrellaceae
Sclerotia appear to have been documented more extensively in Coprinoids than in Leucocoprinus species with numerous common species producing tiny sclerotia with traits similar to those seen in Leucocoprinus species as well as some with far larger sclerotia. Psathyrellaceae are very common finds in plant pots with numerous species across many genera routinely appearing in potting soil. Generally they appear to differ in behaviour from Leucocoprinus species with Coprinoid mushrooms often showing up in freshly moistened soil used to start seedlings whereas Leucocoprinus seem more common in older houseplants.
Coprinopsis lagopus has been documented as producing sclerotia with the purpose attributed to remaining dormant until favourable conditions for germination arise.[41] A study in 1975 on agar cultures described the mature sclerotia as dark brown to black spheroidal structures up to 0.5 mm in diameter with a difference noted between the hyaline interior flesh (medulla) and the outer rind. Sclerotia produced submerged in the agar as opposed to those in the aerial mycelium were noted as being larger at 0.5-1 mm in diameter with an irregular shape and pale brown colour. The rind was suggested as serving as a protective layer for the interior flesh during conditions adverse to normal growth and an extreme outer layer of dead hyphal cells was noted that was thought to be the product of surplus materials during sclerotial develop and presumed not to serve a purpose. This study however considered Coprinus cinereus (now known as Coprinopsis cinerea) to be synonymous with Coprinus lagopus (Coprinopsis lagopus) so it is unclear which species was studied.[42]
When different strains of Coprinopsis lagopus were cultured the majority produced sclerotia with marked differences in the number produced by different strains. Some strains were unable to produce sclerotia and four genes were identified as being involved in the production of sclerotia.[43]
Coprinopsis lagopus has thousands of observations on iNaturalist, though as this species is more commonly known than others it is likely that some observations may be for similar species. There are numerous, morphologically similar or identical species in Coprinopsis sect. Lanatulae[44] but iNaturalist has vastly more observations identified as Coprinopsis lagopus than it does ones left at the section level or identified as any of the other species within this section. Nonetheless there are many observations of Coprinopsis lagopus in plant pots.
Coprinopsis cinerea was isolated from rice husks and when cultured was documented as producing brown, globose to ellipsoidal sclerotia which are 70-180 (200) μm in diameter with a rind comprised of yellow to dark brown pseudoparenchymatous tissue. The internal medulla is comprised of pale brown prosenchymatous tissue.[45]
These are overly not common terms to encounter and indeed this study in some instances uses the term 'pseudochymatous', perhaps erroneously as this yields few results when searched. So it seems necessary to define these terms since they are routinely used in descriptions of sclerotia and speak to the commonality between the features of sclerotia in disparate species.
Pseudoparenchyma is defined by the Collins dictionary as 'a compact mass of tissue, made up of interwoven hyphae or filaments, that superficially resembles plant tissue'.
Prosenchyma is defined by the Collins dictionary as 'a plant tissue consisting of long narrow cells with pointed ends: occurs in conducting tissue'.
This terminology is perhaps not ideal for describing features in fungi rather than plants but they are used commonly enough and they do serve well to distinguish the appearance of the interior and exterior flesh of sclerotia. Whilst both the external and internal tissue of sclerotia that are crush mounted have a highly cellular pattern they are easily distinguished from each other visually by the shape and appearance of this pattern.
Coprinopsis cinerea has also been cultured from rice husks in iNaturalist observation 152704280 by @raingel. Observations for this species are otherwise quite few and none appear to be from plant pots. Similar sclerotia have been documented in Coprinopsis sclerotiger having been isolated from elk dung and cultured on agar[46] however this species is poorly known with only a single observation on iNaturalist at present.
The brown colouration of the external rind in Coprinopsis cinerea may be attributed to the presence of melanin.[47] This may be universal to sclerotia with melanin also noted in other studies and potentially providing the sclerotia with some of their resilient characteristics. The presence of melanin was noted previously in a study on the snow mold fungus, Coprinus psychromorbidus (now known as Coprinopsis psychromorbida) where melanin was found in the outer layer of the rind cell walls via scanning electron microscopy and electron imaging. The black pigment of the mature sclerotia was soluble in KOH, insoluble in water, acetone and ethanol and bleached after 24 hours of immersion in sodium hypochlorite or hydrogen peroxide. A size range does not appear to be given for these sclerotia but the microscopic images show them to be ~500μm in diameter. The optimal temperature for production of sclerotia was 20-25°C, higher than the 10-15°C that was optimal for mycelial growth.[48] In this species then it appears that sclerotia could serve as a means of surviving less than optimal temperatures for growth.
Coprinopsis sclerotiorum is documented as producing irregular, subglobose, dark brown sclerotia that are far larger than others at around 10 mm in diameter which then become 35 x 10 mm and finger shaped.[49] Large sclerotia in a Coprinopsis species have also been documented in iNaturalist Observation 125136438 by @corndog.
A fascinating case of similarly large sclerotia in a Coprinopsis species becoming an industrial contaminant was presented in 1974 when Coprinus stercorarius (now known as Coprinopsis stercorea) was documented producing sclerotia which clogged pipes in the effluent treatment tower of a winery in Australia. The sclerotia had a size of (4) 6-12 (15) mm and circular depressions of 1 mm scattered on the surface rind, which was dull black when dry or shiny black when moist with a white interior when dissected. When moist they had a rubbery texture but became hard and wrinkled after a long period of drying. In a laboratory setting, the mushrooms grew directly from the surface of the large sclerotia with 1-4 per sclerotium. The winery effluent was comprised of spent wash water and lees from the wine making process which had been stored in open dams for 3-4 months before being pumped into the treatment tower. Sodium carbonate was added to adjust the pH to 7 before the liquid entered the tower and urea was drip fed into the liquid to add nutrients to aid bacterial growth. It was hypothesised that the fungus entered the liquid from grazing paddocks surrounding the stored liquid. Sclerotia growth was prevented by removing the urea drip feed. The species was presumed to be synonymous with Coprinus sclerotianus though the sclerotia in that species were described as being 3-6mm in diameter. It was hypothesised that they may have grown far larger owing to the high levels of nutrients provided by the liquid.[50] However considering the numerous Coprinopsis species documented as producing sclerotia it seems possible they may just have been similar species with different sclerotia characteristics.
In 1987 a study on the sclerotia of Coprinus congregatus (now known as Coprinellus congregatus or Tulosesus congregatus) documented the production of sclerotia in liquid culture and noted that the hyphal knots of both the sclerotia and primordia were indistinguishable. The sclerotia formed in liquid culture were noted to be identical to those formed on agar and it appeared that the presence of bacteria, even in low levels that were not easily detected induced the formation. It demonstrated that sclerotia formed in light or dark in liquid culture or agar but that primordia only formed on agar in the presence of light.[51]
These studies on sclerotia in Coprinoids provide a wealth of useful information with many characteristics that are shared between the sclerotia of different species, including some Leucocoprinus species. For example the sclerotia of Leucocoprinus cretaceus develop a dark brown to black surface at maturity which could also be due to presence of melanin. The cellular pattern found in the rind and the internal flesh are similar in appearance so can likewise be described as pseudoparenchymatous and prosenchymatous. These similarities are also found with the sclerotia in other families but are not confined to just sclerotia.
3.2. Sclerotia in Hymenogastraceae
Observations of Hymenogastraceae from plant pots are fairly common on the whole with several Gymnopilus species appearing regularly even in indoor plant pots suggesting a spread via the potting soil or with commercially bought plants. With the notable exception of Psilocybe angulospora which has many observations from potted plants in New Zealand, Psilocybe species don't appear especially common in potted plants though several species do have a few observations each with many more left at the genus level. Agrocybe species also do not seem overly common but some species do show up in plant pots outside. Observations of Galerina or Kuehneromyces species in plant pots do not seem very common. It would appear that whilst many species in this family can grow in plant pots few are likely to spread in this manner given the dearth of observations.
In some observations of Gymnopilus species web like mycelium and small spheres can be seen around the base of the mushroom and on the surrounding substrate. In wild specimens growing from logs many of these are readily explained by the presence of other fungi but in some instances the mycelium does appear to have a similar colour and consistency to L. birnbaumii. However in plant pot specimens whilst there are compelling observations like observation 182132098 by @barefootmark which look very similar to the sclerotia of L. birnbaumii it seems probable that if such structures are sclerotia then they likely actually do belong to L. birnbaumii as in observation 178211973 by @jtanks the two species can be seen growing adjacent to one another in a plant pot. There does not appear to be any documentation on sclerotia in Gymnopilus species.
The Hymenogastraceae family though does contain some well known sclerotia forming species including Psilocybe tampanensis, P. mexicana and P. semilanceata with the sclerotia hypothesised as a mechanism for surviving grassland fires.[52]
In an experiment by Roger Heim the sclerotia of Psilocybe mexicana were found to form more abundantly in darkness whilst the fruiting bodies only emerged if the substrate was exposed to light.[53] This is suggestive of formation underground in nature and with their size and appearance being similar to that of walnuts they do appear to be good mechanisms for survival. P. atlantis is a similar species that also produces sclerotia[54] and P. caerulescens is also said to produce sclerotia[55] however as this species is sometimes cultivated and sclerotia are not reported it is unclear if this is correct. The source cited by "How many fungi make sclerotia?" for P. caerulescens does not provide details on the sclerotia of this species and the sources it is citing for it are not readily available online to check.
The sclerotia size seen in P. mexicana and P. tampanensis would not appear to make for practical transport between plant pots as the large stone like structures would be more readily sieved out or removed and aren't produced in such substantial numbers as to become widely distributed. However as sclerotia have primarily been documented in Psilocybe species due to their desirability to cultivate and as such information dominates search results it is unclear if smaller sclerotia have been documented in other species.
Hypholoma tuberosum was described from mulch beds and compost in Vancouver, Canada. The sclerotia were formed where the soil met the mulch and described as having irregular subglobose, ellipsoid or oblong sclerotia that were often lobed. They measured 85 x 50 x 45 mm and had a tough, dry, brown outer rind with a greyish yellow brown or dark greyish yellow interior and some ochraceous areas.[55] This was compared against Stropharia tuberosa and Agrocybe arvalis as well as the Psilocybe species known to produce sclerotia since these were placed in Strophariaceae at the time.
This species was reclassified as Psilocybe tuberosa in 1998 by Ruben Walleyn having been found in Belgium.[56] However a species was already described under this name in 1904 by Petter Adolf Karsten making this classification illegitimate. Karsten did not described the sclerotia of his species in detail beyond saying that the mushrooms emerged from white tubers in the earth.[57] Based on the description of the mushrooms however this species is thought to be a Psathyrella species though it was never reclassified as such. Subsequently Hypholoma tuberosum was reclassified as Psilocybe tuberifera.[58]
The species has also been documented in Australia as Hypholoma tuberosum with sclerotia measuring 50 x 40 x 20mm in diameter found in a peat and soil mix. The specimens studied by Priest and Simpson came from potted ornamental trees in commercial premises in Sydney after the occupants objected to the presence of the mushrooms which frequently appeared around the plants. Examination determined that the mushrooms were fruiting from the sclerotia which were associated with coarse pieces of peat. Additional specimens were collected in dry areas of peat bog and from sandy margins of a creek. In this instance sclerotia and fruiting bodies were only found in sand deposits directly adjacent to the water. The study also noted that in the vicinity of the Georges Creek by which the sclerotia were found there were numerous commercial nurseries. It was hypothesised that the specimens found growing beside the water were the result of sclerotia that had been screened out of peat by a commercial nursery located within the watershed. Commercial peat in the region was derived predominately from sedges rather than sphagnum though sphagnum based peat had been imported in the past from Europe or New Zealand. A connection to Canada via imported peat or potted plants was not found. The sclerotia were potted in soil in a greenhouse and germinated to produce mushrooms.[59]
The authors of the study questioned what role sclerotia served in nature and it was considered that if the peat were the natural habitat of H. tuberosum then the sclerotia would serve as an ideal mechanism to store nutrients and survive the dry periods in which mycelial growth would not be possible.[59]
Hebeloma sacchariolens is documented as having conspicuous white sclerotia which are globose, subglobose or flattened and 200-400 μm in diameter. These are found adhered firmly to the mantle surface of the mycorrhiza. The same study also documented sclerotia in Paxillus involutus but described these as loosely connected to the mycorrhiza.[33]
The sclerotia of H. sacchariolens lack the dark pigment and thick rind of many other species with only a thin wall surrounding an internal pseudoparenchymatous structure containing large amounts of lipids, which are hypothesised to serve as a store of energy. When sclerotia were detached from host roots and buried in moist soil they remained viable for at least 40 weeks and when introduced to birch roots after this time were still able to develop mycorrhiza and exclude other mycorrhizal fungi. Sclerotia examined after being buried in soil for 16 and 40 weeks were noticeably softer though retained their white colouration and structural integrity. After inoculation from sclerotia new sclerotia were observed to have grown within the 16 week period of the study.[60]
There are a few observations of Hebeloma species in plant pots on iNaturalist but as they are mycorrhizal these are of course only where trees are planted in pots. However it may also be interesting to note that the gasteroid genus Hymenogaster are very similar in appearance to the sclerotia of Hebeloma sacchariolens with their smooth exterior surface and an interior which has a similarly pseudoparenchymatous appearance. Hymenogaster are not sclerotia however and this internal structure is a sporulating surface. The Psilocybe genus likewise contains some gasteroid species such as Psilocybe weraroa which when bisected has an appearance vaguely similar to the fruiting bodies of Hymenogaster species and the sclerotia of Hebeloma sacchariolens. Since sclerotia and fruiting bodies both emerge from hyphal knots the similarities in appearance are intriguing. Tympanella galanthina is a sectoid fungus in the Hymenogastraceae family which also has some observations in plant pots in New Zealand.
In the Ascomycete family Tuberaceae an evolutionary lineage between surface fruiting bodies and truffles has been hypothesised whereby arid conditions selected for fruiting bodies that were more enclosed, so as to reduce water loss. According to the theory, this selective pressure then lead to the loss of the ability to forcibly discharge spores resulting in distribution of spores via animals instead. Further selection along this line resulted in truffles which fruit below ground and contain a solid gleba containing spores.[61] Given the similarity in appearance between some of the sectoid or gasteroid fruiting bodies and sclerotia in the Hymenogastraceae family it may be interesting to consider a similar evolutionary forcing.
The sclerotia of Agrocybe arvalis are evidently quite large and readily found with numerous iNaturalist observations documenting them including observation 35811272 by @heelsplitter, 146456608 by @hiroko_s and 95141398 by @leptonia. Whilst Agrocybe species are sometimes documented in plant pots these appear to be in outside pots where introduction via spores over time is more likely than the fungus having come with soil. There are currently no observations of Agrocybe arvalis from plant pots and sclerotia of this size would not seem like such an ideal distribution mechanism between plant pots.
Agrocybe arvalis has a taxonomic history which includes several basionyms as the species is evidently common enough to have been described independently several times. The specific epithets for several of the synonyms indicate that the sclerotia were the typifying feature. Naucoria tuberosa was reclassified as Agrocybe tuberosa while Galera arvalis var. tuberigena was reclassified as Agrocybe tuberigena and Naucoria arvalis var. tuberigena. The description for Naucoria tuberosa notes that the mushroom emerged from a hard, almost spherical, wrinkled, black sclerotium that was about 1.5-2cm in diameter with a white interior when cut.[62] The sclerotia were not documented in the original description of Agaricus arvalis.[63]
Due to their similar appearance some genera in Hymenogastraceae were previously classified as Strophariaceae and confusion can still occur when identifying them. This is also seen in some accounts of the sclerotia like structures in Strophariaceae.
3.3. Sclerotia in Strophariaceae
Stropharia species do not seem to be a regular occurrence in plant pots with no definite observations on iNaturalist collected to date besides cultivated Stropharia rugosoannulata. Strophariaceae do not seem to be typical to find in plant pots in general. Leratiomyces ceres appears to have more observations from plant pots than everything else in the family combined, however observations of it from plant pots are few and far between when browsing through the thousands of observations of this species. Sclerotia, or structures similar to them have been documented in some species in the Strophariaceae family although they appear to be considerably larger than the small sclerotia common amongst the Coprinoids and Leucocoprinus species and as such are unlikely to be readily transferred in potting soil.
Stropharia tuberosa was described in 1918 by Henry Curtis Beardslee in Curtis Gates Lloyd's Mycological notes. Specimens were found growing in masses of old cow dung in the woods in West Virginia. They were compared to Stropharia umbonatescens and S. mamillata but differentiated by the spore size as well as the sclerotium, which every specimen of Stropharia tuberosa was found to emerge from. A size range is not given for the sclerotia but the photograph shows a large solitary sclerotium which appears ~2/3 the size of the cap of the mushroom, the size range for which is given as 2-4 cm. From this photo then the sclerotium appears to be ~1.3-2.6 cm wide. The thickness of the stipe is given as 3-4mm[64] which by chance are the widths I find from measuring the bottom of the stipe on my screen at the default resolution and fullscreen view from the source on the internet archive. Measuring the sclerotium in the same manner produces a size of ~1.5-2.3 cm.
Lloyd notes that Naucoria scleroticola which was also found in West Virginia and published in an earlier edition of Mycological notes appears similar and that since its publication Jakob Emanuel Lange had communicated that Naucoria arvalis also sometimes developed from sclerotia.[64] Naucoria arvalis is now known as Agrocybe arvalis and whilst Naucoria scleroticola has not been reclassified nor was it ever formally described as such as it was considered to be so similar in appearance to Naucoria semiorbicularis as to potentially simply be the same species, only growing from a sclerotium. As such the 'description' of this species specifically states that they are not describing a new species and it was thought that the sclerotium could be an occasional occurrence. The specimens were sent to Lloyd by Reverend Boutlou who noted that all of the Naucoria in his garden had sclerotia and that these ultimately dried up and became hollow leaving only hard skin behind.[65]
Naucoria semiorbicularis is now known as Agrocybe pediades and so it appears that this was an account of sclerotia in an Agrocybe species however Stropharia tuberosa was considered distinct as it differed in the gill colour amongst other features. Stropharia tuberosa was reclassified as Protostropharia tuberosa in 2014 however as this was only an Index Fungorum nomenclatural novelties update which reclassified four species as Protostropharia no additional information was provided on the species or the sclerotia produced by it.[66] The holotype of Protostropharia tuberosa may be the only preserved specimen that exists and the species is considered rare and poorly known. The 'sclerotia' formed by it may in fact be pseudosclerotia with similar structures sometimes found in Protostropharia alcis ssp. austrobrasiliensis. Pseudosclerotia were not observed with P. dorsipora or P. semiglobata.[67]
These are the two most commonly observed Protostropharia species on iNaturalist and whilst it is likely that P. semiglobata contains observations for other species due to it simply being the most commonly known, other species in this genus do not have many observations. Observations of Protostropharia alcis on iNaturalist are relatively few but frequently show mushrooms growing from elk or moose dung such that they have a similar appearance to the mushrooms growing from sclerotia however it is unclear if such structures are contained within the dung or if the similarity is simply coincidental. Regardless it could make documentation of the sclerotia, or pseudosclerotia in these species less common due to people either dismissing them as dung or being unwilling to dissect said dung in order to explore further.
Pseudosclerotia are also documented in Protostropharia luteonitens with these structures distinguished from sclerotia by the presence of pieces of substrate within the medulla, or internal flesh. The manner in which sclerotia and pseudosclerotia form is distinct with sclerotia forming from hyphal knots in the same way that mushrooms form whereas pseudosclerotia are cut out from the substrate by pseudosclerotial plates.[68] In wood rotting species like Armillaria mellea pseudosclerotial plates are the 'zone lines' seen in a cross section of infected wood with these lines representing a sectional view of the outer rind of the forming pseudosclerotial body.[69] A simplistic way to conceive of the difference may be to consider a sclerotium as forming and displacing the substrate as it grows outwards whereas pseudosclerotia subsume the substrate as they grow inwards such that it becomes incorporated into the pseudosclerotial mass.
The hyphae inside the pseudosclerotia may then continue to digest the substrate resulting in partial or complete replacement of the substrate with hyphae. Subsequently the volume of substrate contained within pseudosclerotia varies and if no substrate remains then it may not be possible to distinguish them from sclerotia. The pseudosclerotia of Protostropharia luteonitens are described as almost black, with an irregular bean or potato shape measuring 8-15mm long and white interior flesh composed of compact hyphal masses. The exterior rind is composed of thick walled, dark brown polyhedral cells and measures 25-40 μm thick but is brittle making it hard to section. The rind does not dissolve in 10% sodium hydroxide though a crush mount in this reagent reveals the presence of small crystals. When sulphuric acid is applied needle like crystals are formed indicating calcium content. The rind stains weakly with Congo red but is unreactive with many other stains including several blue stains and Melzer's reagent. Sclerotia were not mentioned in Lucien Quélet's 1888 description of Stropharia luteonitens though he did note occasional sclerotia in Stropharia stercoraria (now known as Protostropharia semiglobata). This study also suggests that the differentiation in aerial and submerged sclerotia observed in cultures in Coprinus cinereus may represent the presence of both sclerotia and pseudosclerotia.[68] The example given is for Coprinus cinereus however the citation is for the 1975 study on Coprinus lagopus, though this study did consider the two species synonymous.
Considering that pseudosclerotia are likely even less well known than sclerotia and that their irregular appearance, dark exterior surface and the pieces of plant matter that they contain can give them an appearance like dung, especially where large amounts of partially decomposed plant matter is present, it seems possible they may be under-reported. After reviewing this material I made a chance observation of structures which appeared to be pseudosclerotia in a wormery which had been started from a contaminated tub of cultivated Ganoderma lucidum. The structures had a similarly thin, brittle dark exterior surface as described here with a distinctive crunch when broken. The material contained within them was the only material in the wormery that had not been consumed by the worms, isopods, springtails and other creatures living in the substrate. Whilst the exterior was thin and brittle it appeared effective at keeping these creatures out enabling some mycelium to survive within. These structures would easily have been overlooked if this review had not been conducted prior to collecting material from the wormery as it would not have been clear what they were.
3.4. Sclerotia in Pleurotaceae
Pleurotus tuber-regium is documented as producing rather large, edible sclerotia which are consumed in Nigeria and readily available to purchase in markets. Subsequently there are many papers about the sclerotia in this species (often under the name Pleurotus tuberregium) regarding nutritional content, medical value and cultivation but there does not appear to be documentation of sclerotia in other Pleurotus species. It is regarded as the only known sclerotia forming species in the Pleurotus genus as such its classification in this genus is questioned.[70]
The sclerotia are globose to ovoid and can reach 30cm or more in diameter. In studies on the nutritional content, potassium was found to be the most abundant mineral in the cultivated sclerotia, in most cases followed by sodium with the magnesium, calcium and phosphate content varying by substrate. Sclerotia production started in culture 5-6 weeks after spawning and growth continued until they were harvested 14 weeks after spawning, by which point they had turned light brownish. Sclerotia remained viable and able to produce fruiting bodies after 9 months storage in the refrigerator at 12°C.[71] iNaturalist contains many observations of this species, especially from Australia and the large sclerotia are frequently visible on top of the leaf litter or wood with mushrooms growing directly from them.
Pleurotus species do not seem to be commonly observed in plant pots, except in cases where cultivated substrate has been buried however there are many observations on iNaturalist of Hohenbuehelia petaloides in plant pots. Potentially this is another case of this species being more well known than others such that observations for it actually include those for several species but such observations do at least seem to be for Hohenbuehelia species. They appear relatively common to find in indoor plant pots suggesting spread via the potting soil.
3.5. Sclerotia in Galeropsidaceae
Panaeolus species appear to be reasonably common to find in plant pots with Panaeolus cinctulus being the most common ID given, though whether these IDs are correct or the result of this species simply being more well known than others is not clear. Sclerotia or pseudosclerotia are sometimes observed in cultivated P. cinctulus and they appear to contain psilocybin given the blue colouration, resulting in them sometimes being quite noticeable to observers. Similar observations exist for sclerotia in P. tropicalis and possibly some otherwise unnamed Panaeolus species that have been cultivated. However sclerotia do not appear to have been formally described or studied in these species and are not observed universally so the nature of these structures is unclear.
Sclerotia have been described in Panaeolus subbalteatus with greenish blue sclerotia forming abundantly when cultured on agar. When established mycelium was transferred to a Petri dish of malt agar or other mediums, large drops of exudation appeared after five days along with sclerotia. When first observed these were initially thought to be contamination by Penicillium owing to the striking blue green colour however examination revealed them to be sclerotia. They were spherical and ranged from 1-4mm with some as large as 6mm. As they matured they became hard and darker in colour. The sclerotia were produced on both diploid and haploid mycelium with the diploid sclerotia only producing diploid mycelium and never haploid. The sclerotia were able to produce mycelium even after drying for a month but fruiting bodies did not emerge from the sclerotia.[72]
3.6. Sclerotia in Bolbitiaceae
Conocybe and Pholiotina species appear to be very common to find in plant pots but despite Bolbitius species having almost as many observations on iNaturalist as Conocybe they do not seem to occur often in plant pots. There does not appear to be any documentation on sclerotia for this family besides mention of Pholiotina cyanopus (formerly Conocybe cyanopus) producing sclerotia,[52] with more attention paid to this species and it's cultivation because of its psychoactive properties. This trait however makes researching it complicated as there are a vast number of sources stating that it produces sclerotia but none appear to provide a description, photos or evidence of them. Additionally such searches routinely turn up information on the sclerotia in the Psilocybe species instead with Pholiotina or Conocybe cyanopus merely being mentioned alongside them.
iNaturalist contains few observations of this species and sclerotia are not apparent in any of them however this is not surprising as if they are anything like the sclerotia in Conocybe cf. macrospora they may not be apparent macroscopically.
 |
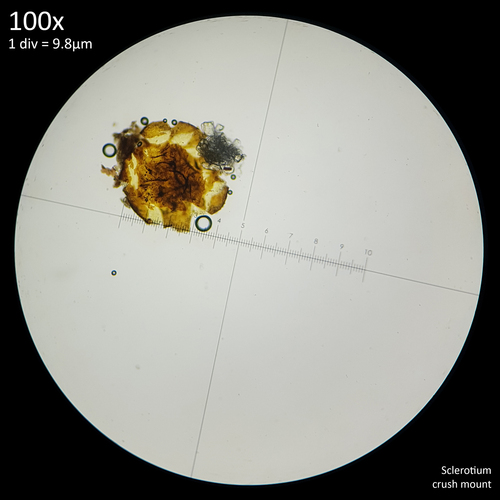 |
|---|---|
| Fig. 8 - Sclerotia stuck to stipe | Fig. 9 - Sclerotium crush mount |
A Conocybe species was found growing in an outside plant pot in late September in the United Kingdom and examination of the spores resulted in a size range of 13-21 x 8-10 µm which appears to match the description of Conocybe macrospora. When viewed at 40x magnification small clusters of irregularly shaped, roughly ellipsoidal brown structures were found (fig. 8). Isolating them from the stipe was not trivial owing to the minute size that made simply losing them easy. When specimens were placed on a slide they were barely visible to the naked eye and appeared like specks of dust on the illuminated glass and it would be easy to dismiss them as such. Crush mounts of these specimens (fig. 9) however resulted in the familiar appearance seen in crush mounts of Leucocoprinus sclerotia personally observed as well those seen in photos of sclerotia in Coprinopsis species.
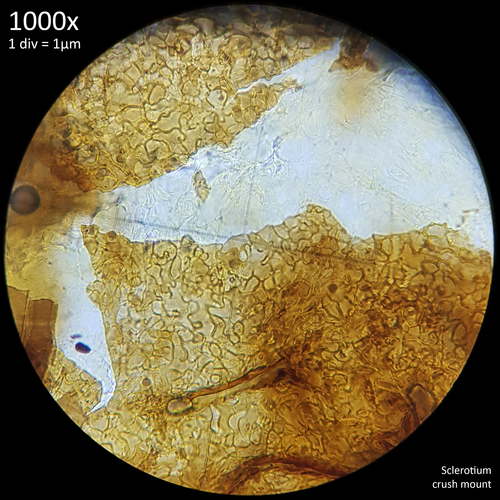 |
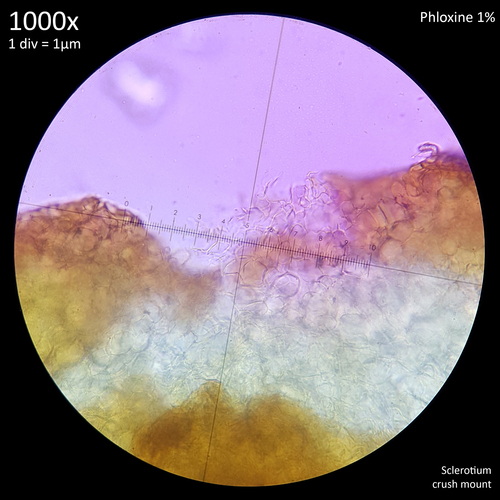 |
|---|---|
| Fig. 10 - Pseudoparenchymatous rind | Fig. 11 - Prosenchymatous internal flesh |
A more detailed look revealed the same brown, pseudoparenchymatous appearance of the rind (fig. 10) with hyaline prosenchymatous internal flesh spilling out when crushed (fig. 11). The sclerotia did not seem to offer any noticeable resistance to crushing so their hardness could not be gauged however they were so small that it is to be expected that they should not offer as much resistance as the larger sclerotia found in other species. Due to the limited number of specimens found and the extremely small size of them making manipulation awkward no measurements were attempted of the intact sclerotia with it instead being prioritised to mount them before they became lost. From the photos taken however the specimens all appeared to be under 125 µm with the smallest observed being perhaps half that size.
It will be necessary to culture this species to confirm that the sclerotia belong to it however given their proximity to the mushrooms it seems probable that they are related. In any case it may be prudent, based on this observation, to look for similar when examining Conocybe specimens. Without specifically looking for sclerotia there would scarcely be a reason to spend the time and effort to isolate and crush such objects from the base of the mushroom as in most cases such objects could be presumed to be sand, soil particles or frass. It seems probable that other species may produce similarly tiny sclerotia which simply go overlooked and only become obvious in cultures.
3.7. Other species
Cortinarius calochrous (now known as Calonarius callochrous) is ectomycorrhizal so is not going to be a species which occurs with potted plants. However the sclerotia still appear to have some similar traits as they are up to 1mm in diameter, subglobose to oblong and sometimes bi-lobed. Beneath the mycelial coating they are smooth with a white surface and a hyaline, pseudoparenchymatous interior that is described as containing subglobose, rectangular or triangular cells with a size range of 6-19 x 3.6-16 μm. In KOH they develop a pink colour. Kernaghan notes that sclerotia production by ectomycorrhizal basidiomycetes is common in Boletales but that sclerotia have also been documented in Hebeloma crustuliniforme, H. sacchariolens, Cortinarius subporphyropus (now known as Thaxterogaster subporphyropus), Cortinarius magellanicus and Cortinarius alnobetulae (Calonarius alnobetulae).[73] Given the very large size of the Cortinariaceae family and their ectomycorrhizal growth habit these are ultimately beyond the scope of this investigation and may be best explored separately.
Another mycorrhizal species which has been documented as producing sclerotia is Inocybe aeruginascens which produces abundant greenish-blue sclerotia in culture which have a diameter of 1-2mm. Sclerotia formation started 5-9 days after culturing with exudation of water droplets noted during formation. They start light green before quickly turning dark green and finally greenish-blue[74] and the sclerotia were found to contain 0.1% psilocybin by dry weight. This was documented in culture but ultimately degenerated after a few months, presumably due to the lack of a mycorrhizal symbiont.[75]
This appears to be another case where a species was studied and cultured due to the psychoactive properties whilst other species in the genus may not have been studied in such detail. There does not appear to be documentation on sclerotia in any other Inocybe species.
Willetts lists Typhula gyrans, T. intermedia, T. sclerotioides as producing sclerotia though many more species in this genus are documented as producing sclerotia including (from a cursory search) Typhula incarnata and T. ishikariensis [76], T. variabilis, T. laschii and T. japonica[77]. Typhula suecica is a species with fruiting bodies that grow from single sclerotium that are smooth, lenticular to ovoid and 600-1300 x 400-700 µm. When fresh they are reddish brown but dry to a dark reddish brown.[78]
Whilst Typhula are placed within the Agaricales order these would seem to be generally beyond the intended scope of this study. As they are plant pathogens these species have already been very well studied and documented in order to combat them and as such it does not seem necessary to collate and summarise the vast quantities of information on them here. These studies may contain some relevant information to study of sclerotia in general however. For instance the methodology of culturing sclerotia by soaking them in distilled water for 20 hours at 4°C before surface sterilising them with 70% ethanol for 5 seconds and sodium hypochlorite for 5 minutes before rinsing multiple times with sterile water and placing onto agar.[76] The resilience of sclerotia to chemicals which makes this kind of surface sterilisation possible, whilst perhaps detrimental for efforts to combat plant pathogen species in fields, may be useful for culturing them.
In Agroathelia rolfsii (formerly known as Sclerotium rolfsii) the dried sclerotia have been shown to become vulnerable to colonisation by other micro-organisms resulting in rot within 2 weeks when dried sclerotia were remoistened in soil. However drying also stimulated germination and it was hypothesised that in nature drying main be the main mechanism that promotes germination.[79] It may therefore be interesting to see whether the sclerotia in saprotrophs share traits with the sclerotia producing plant pathogen species.
Collybia tuberosa was described in 1871 by Paul Kummer as a tiny all white mushroom smaller than a penny with a stem about 1 cm tall which grows on mosses, large mushrooms, leaves, etc. No description is given of the sclerotia besides that the base is thick, hard and bulbous with a tuber.[80] William Murrill wrote that the sclerotia are bioluminescent.[81]
The species was first described and illustrated by Pierre Bulliard in 1786 as Agaricus tuberosus. Bulliard described the species as originating in the form of small seeds which grow larger and elongate ultimately leading to mushrooms growing from them. The tuber is described as solid with a taste like scorzonera, presumably referring to Pseudopodospermum hispanicum (formerly known as Scorzonera hispanica). The accompanying illustration shows the 'seeds' in multiple stages of growth with and without mushrooms and has them emerging from the gills of a decaying Agaric.[82]
Describing them as seeds truly does seem apt given the appearance of them seen in observation 131625877 by @kallampero and 178773631 by @ksanderson. This growth behaviour is seen in many of the iNaturalist observations of this species in which the sclerotia are abundantly visible however Collybia cookei and C. racemosa are also documented as producing similar sclerotia and Komorowksa notes that distinguishing these species is troublesome even via microscopy. Identification is aided by examining the cortext of the sclerotia with surface material of fresh or dry sclerotia crushed in 3% KOH or NaOH and phloxine. The surface texture of each species is distinct with C. tuberosa having elongate hyphae on the surface of the sclerotia whilst in C. cookei they are round and in C. racemosa they are angular. C. cirrhata does not produce sclerotia.[83]
C. racemosa is now known as Dendrocollybia racemosa and sclerotia are likewise readily seen in iNaturalist observations of this species. There are no observations on iNaturalist from plant pots for any of these species or anything else in the Collybia genus. This is not surprising given their tendency to grow from decaying mushrooms though possibly the sclerotia of these species provide some explanation for the early descriptions of mushrooms arising from sclerotia on the gills of decaying Agarics in bark beds. The similar looking (and similarly named) Lactocollybia genus does have several observations from plant pots, specifically from potted Orchids. However these are not closely related with Collybia placed in Tricholomatineae and Lactocollybia in Marasmiineae.
Nidulariaceae are not uncommon to find in plant pots and sometimes appear alongside L. birnbaumii. Macroscopically their peridioles are somewhat similar in appearance to sclerotia but of course they contain spores whereas sclerotia do not. iNaturalist observation 150248388 by @komille277 of a Mycocalia species shows a similarly pseudoparenchymatous surface texture when the peridioles are viewed at 1000x magnification. However unlike the sclerotia they appear to readily take up a blue stain. The peridioles of many Nidulariaceae have a brown exterior similar in appearance to sclerotia though whether this is also due to the presence of melanin does not appear to have been discussed.
It has been hypothesised that the Nidulariaceae may engage in 'seed mimicry' with their peridioles appearing like seed to foraging birds who may then eat them resulting in spores being distributed further afield than could be achieved just from raindrops splashing the peridioles out of the cup.[84] This has not been demonstrated in nature but one study demonstrated that canaries would eat them only if mixed with other food and undamaged spores were able to pass through the digestive system.[85]
There is an association regarded between many bird and fungi species with spore distribution via bird faeces proposed as a dispersal mechanism as well as an explanation for why New Zealand appears to have so many brightly coloured gasteroid fungi and truffles. Many of these species resemble berries with their red, blue or purple colouration and the region has many ground dwelling birds[86]
An association between birds and sclerotia however does not seem to have been considered, perhaps because sclerotia are generally regarded as large subterranean structures with the small, seed like sclerotia of Leucocoprinus and Coprinoids being less well known. Given the seedlike appearance of the tiny sclerotia found in these species it seems worth considering whether they can also be spread via birds.
4. Conclusion
Documentation of sclerotia in the Agaricales appears lacking and it seems probable that if more species are examined with a specific view to looking for sclerotia then this trait will be found in other species. As is seen with the sclerotia in Leucocoprinus, Coprinopsis and Conocybe these structures can be so small as to go unregarded in nature as they are not readily distinguished from pieces of debris, dust, sand, soil, eggs or insect frass unless the observer is deliberately looking for them. These tiny sclerotia do not seem to be well known and so most observers are not likely to notice them and in the case of the smallest sclerotia observers without access to a microscope are not going to be able to correctly diagnose the structures as sclerotia. It is only with a crush mount or dissection that their true nature is revealed and dissection is not trivial owing to the size and hardness of the sclerotia.
Realistically the only way to observe and study these tiny sclerotia seems to be to culture them. Whilst they are hard to find in nature they can be abundantly apparent on agar or other substrates such as brown rice. Additionally these nutritionally rich substrates generally appear to result in the production of vastly more sclerotia than may be found in nature. As sclerotia have shown some resilience to contamination and as their production was only observed in conjunction with some manner of contaminate in a number of studies it is possible, in some species at least, that sclerotia production might be unreliable in a sterile substrate. This will require further examination but my own initial studies do seem to suggest that sclerotia growth in Leucocoprinus species may be prompted by (though not reliant upon) the presence of contaminants and that the sclerotia seem to afford some protection against them. Subsequently if a species is cultured on agar or substrate in a sterile environment and no sclerotia are produced this may not necessarily guarantee that the species is incapable of producing them.
It is my hope that by studying the sclerotia produced by Leucocoprinus species in culture their purpose in nature may become clearer. To that end I intend to document the sclerotia of some of the most commonly observed species from plant pots and explore their possible role as a distribution mechanism for the fungus in their natural environment. It is my expectation that the presence of sclerotia as well as the specific traits of them may explain why some species are so common in plant pots and this in turn may help understand their behaviour in the wild.
References
Journal of Japanese Botany, Vol. 28, No. 3, pp. 70-72.














