A preliminary investigation of sclerotia in Leucocoprinus cretaceus
Abstract
Sclerotia of Leucocoprinus cretaceus were observed growing in agar cultures and cultivated in various substrates with abundant sclerotia growth always preceding any mushroom development. The sclerotia are roughly spherical, ovoid or elliptical and range in size from (135) 245 - 735 (1150) µm with a mean average size of 475 x 413 µm. Immature sclerotia have pale yellow gelatinous flesh encased in a web like mycelial envelope similar to those of Leucocoprinus birnbaumii however at maturity they develop a hard, dark brown or black rind and become free from the mycelial envelope. The sclerotia are hard, durable structures that require force to cut or crush and new mycelial growth is easily cultured from loose sclerotia on agar, in solid substrates and in liquid. Sclerotia development can start within 1-2 weeks of inoculating a substrate and new sclerotia are produced constantly as the mycelium spreads with high nutrient substrates ultimately becoming filled with hundreds of thousands of sclerotia. The vast numbers of sclerotia appear to provide some resilience against mold and mycophagous pests but may also suggest that they serve not just as a means of surviving adverse conditions but also as an effective delivery mechanism for propagating the fungus. Sclerotia are readily separated from the substrate in clusters or individually and are prone to adhering to surfaces. Before losing the mycelial envelope the sclerotia are extremely hydrophobic resulting in them floating away in water and clinging to anything solid which could serve as a potential means of distribution in tropical rainforests via flood water. Considering the seed or insect egg like nature of the sclerotia as well as the very strong smell of the myceliated substrate possible interactions with animals including birds, termites and ants are also explored.
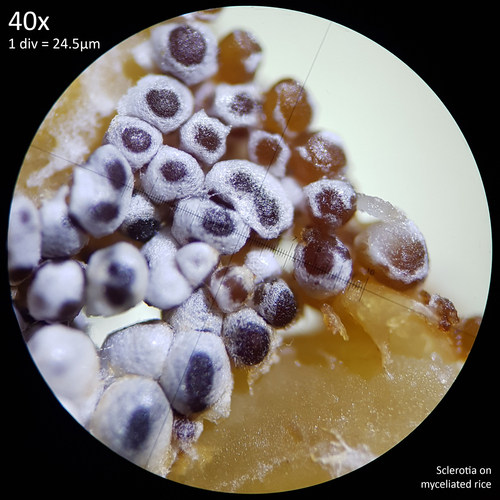
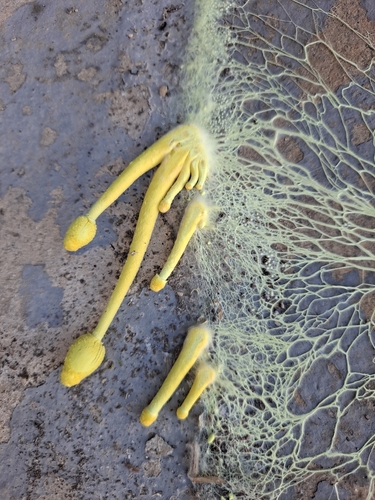
Fig. 1 - Maturing sclerotia of Leucocoprinus cretaceus cultured on brown rice.
1. Introduction
Sclerotia are a commonly observed feature in Leucocoprinus birnbaumii and are routinely present in observations of this species from houseplants either with or without the associated mushrooms. It seems probable that the abundant sclerotia assist this species in spreading via potted plants and compost as they would be easily transferred between pots when plants are transplanted or repotted.
Leucocoprinus cretaceus is also found in plant pots all around the world however to date fewer than a hundred observations of this species from plant pots have been collected on iNaturalist as opposed to the thousands from L. birnbaumii. Even taking into account the likelihood of multiple species being identified as L. birnbaumii it is still substantially more common to find in plant pots.
Culturing Leucocoprinus cretaceus has demonstrated that it also produces very large quantities of sclerotia and is able to grow well in substrates with multipurpose compost and coir. This could therefore call into question the presumed role the sclerotia play in facilitating spread through plant pots. ie. If both species favour the warmth, produce sclerotia abundantly, grow well in potting soil and have global distribution then why is one species far more common in plant pots?
It is possible that the answer lies in the observable differences between the sclerotia of these species and the differences in the growth behaviour. However without knowing what functionality the sclerotia provide these species in nature it would seem difficult to know how they function in plant pots. The more intriguing questions therefore would be what role the sclerotia play in the survival of these species in nature and what natural conditions is the artificial environment of a potted plant simulating?
In captivity the sclerotia of Leucocoprinus cretaceus have proven to be an extremely effective means of propagation. The vast numbers that they are produced in and the properties they possess are suggestive of a role in nature not simply as a means of laying dormant and surviving but actively spreading through the environment and exploiting the unique ecological niches of their native habitat.
The following study was initiated prior to carrying out a detailed review of the literature on sclerotia in Leucocoprinus species with the review conducted in order to see which species sclerotia had previously been documented in. The findings are presented in A review of the literature on sclerotia in Leucocoprinus species and other Agaricales. Conducting this review after having made so many observations of the sclerotia in Leucocoprinus cretaceus proved very advantageous with many 'aha' moments upon finding similar traits noted in other species. However no previous documentation of sclerotia in Leucocoprinus cretaceus was found and so this trait may not have been discussed previously with this species. The most comprehensive study on sclerotia in Leucocoprinus species appears to be Oreste Mattirolo's 1918 work however Mattirolo was unfortunately not successful in culturing these species due to contamination and he did not find sclerotia in L. cretaceus, stating that it did not produce them.[1]
2. Sclerotia in Leucocoprinus cretaceus
Sclerotia in L. cretaceus initially appear similar to those of L. birnbaumii, only without the yellow mycelium, but they soon darken and develop a brown/black colour as they mature. This darker colouration becomes apparent as they start to emerge from the mycelial envelope (fig. 1). At this stage of development they are small, hard structures with an approximate size range of 245 - 735 µm. The larger figures in this range tended to represent the length of non-spherical sclerotia with a narrower width. The average size was around 440 - 490 µm from samples measured growing on rice and agar which either still had the white mycelial envelope or were starting to lose it showing the black surface below.
If they are isolated and have enough space to grow they form almost spherically or are ovoid or elliptical with relatively symmetrical proportions. However in the most densely clustered growth in which maybe 50 sclerotia were found on a single grain of the brown rice substrate they deform where they touch each other. Some are left with almost geometric looking shapes and flat edges. Subsequently measuring in situ is complicated by sometimes being unable to tell where one ends and another begins and in isolation by the non uniform shape with flattened surfaces.
 |
 |
|---|---|
| Fig. 2 - Mature sclerotia | Fig. 3 - Dissected sclerotia in 70% isopropyl alcohol |
When sclerotia were again harvested from this brown rice substrate one month later as well as from a jar containing a soil substrate they were almost completely black having lost the mycelial envelope (fig. 2). The shapes appeared more irregular and shrivelled as if they had shrunk when drying. 70 sclerotia were isolated and measured with a range of 270 - 685 x 270 - 610 µm. The mean value was 475 x 413 µm, the mode was 490 x 367.5 µm and the median 465.5 x 416.5 µm. One larger specimen was found that was excluded from this range as it appeared to be two sclerotia joined in the middle with a narrower portion. This was 735 µm long with a width of 415 at one end and 345 µm at the other. Other specimens were found that appeared as if two sclerotia had formed together hemispherically with a distinct line where they touched which gave the appearance of a coffee bean. The larger measurements in the initial count of the less mature specimens may have been sclerotia that were likewise stuck together with this fact hidden by the mycelial envelope.
Application of 70% isopropyl alcohol aided in the dissection of the mature sclerotia and revealed a yellow interior which is clearly distinct from the darker exterior surface (fig. 3). However a colour change was noted in the isopropyl alcohol with the black exterior becoming more reddish brown with gold flecks and such a deep yellow interior was not so noticeable with or without other reagents. This exterior dark layer measured ~5-10 µm thick and was not so readily distinguished and measured without the application of the isopropyl alcohol.
In high nutrient substrates sclerotia are produced quickly and in great abundance. When a sterile substrate jar consisting of 60g brown rice and 90 ml rainwater was inoculated from liquid culture and left at room temperature sclerotia development was observed 9 days later and the substrate was deemed to be fully colonised by mycelium 23 days after inoculation, though new sclerotia growth continued even as mushrooms developed. The majority of the substrate was dissected 4 months after inoculation with some small amounts having been removed along the way as mushrooms were harvested or as donor material was taken to inoculate additional substrates.
The sclerotia appeared most densely clustered and numerous on the exterior surface though were also found throughout the entirety of the 7cm diameter x ~6.5 cm height substrate. Breaking up the myceliated rice by hand and sieving it was sufficient to separate the majority of the sclerotia though many remained in amongst the rice and were not readily removed without accumulating more rice debris. Based on appearance, perhaps around 10% of the sclerotia remained in the rice and the recovered sclerotia also contained some fine debris. Additionally some dark brown to black irregularly shaped clumps of hard material that measured 0.5-1cm were found amongst the rice and were encrusted with numerous sclerotia that could not be easily removed. These clumps may have been pseudosclerotia and appeared to have their own brittle rind, though they were so densely packed with sclerotia that at this level of maturity it was difficult to distinguish any features. Sclerotia were not recovered from these structures though the brittle rind of the pseudosclerotia did contribute to the debris mixed in amongst the collected sclerotia.
Before drying the collected material had a volume of approximately 35ml and weighed 19g, though some drying occurred during the time it took to collect the material. After one week of drying in the open air this material dried to approximately 10.5g. Some obvious debris like tiny immature primordia were removed manually and a minimal amount of larger material that did not pass through a 955-1,029µm mesh was discarded as debris as few sclerotia were present. The remaining material was then passed through a series of mesh screens and separated into size ranges. The meshes were measured under the microscope to find the range in hole sizes since they differed quite greatly to the size they were sold as.
The 0.875g of material which passed through the 294-343µm mesh contained a lot of fine debris and powder which made it impractical to estimate a quantity of sclerotia. The smallest sclerotia found in this material were ~150 x 135 µm and appeared to have the colouration of mature sclerotia. The 1.235g of material which passed through the 955-1,029µm mesh but was caught by the 637-686µm mesh appeared to be approximately half debris so was likewise not included in the estimate.
Microscopic examination of this material found some sclerotia that were much smaller than this range though clustered together or stuck to the debris. Some uniformly shaped sclerotia in the range of 800-900 µm were found in this material but many others were irregularly shaped conjoined pairs of sclerotia or clusters that appeared like several sclerotia that had formed together and merged. The largest sclerotia found ranged from 1,000-1150µm though many of these were highly irregular in shape and clearly the result of numerous sclerotia merging together so firmly as to become inseparable. A few were found with roughly uniform proportions which may have been single sclerotium though some appeared segmented and tightly stuck together such that it was uncertain.
Debris was also present in the other size groups though was much less significant and represented only a small amount of the total material. The bulk of the material was larger than 294-343µm and smaller than 588-637µm. This was divided into three size groups (table. 1) with the 2.74g, 2.415g and 2.88g of each group containing mostly sclerotia with minimal debris. For each of these three groups five 0.1g (±0.005g) samples were weighed out, the material was then poured onto a white plastic tray in small portions at a time and manually counted with the naked eye. One difficulty with this approach was that some sclerotia appeared like clusters with irregular shapes and when rolled between the fingers would break up into numerous small sclerotia. Others that appeared similar however would not break up and may have been conjoined. As such the counts could not be expected to be exact.
| Minimum mesh size | Maximum mesh size | Weight collected (±0.005g) | Sclerotia quantity in 0.1g (±0.005g) sample | Estimated quantity range | Estimated quantity average |
|---|---|---|---|---|---|
| N/A | 294-343µm | 0.875g | N/A | N/A | N/A |
| 294-343µm | 441-490µm | 2.74g | 1,954 - 2,438 | 53,539 - 66,801 | 60,860 |
| 441-490µm | 490-539µm | 2.415g | 1,315 - 1,684 | 31,757 - 40,668 | 35,201 |
| 490-539µm | 588-637µm | 2.88g | 828 - 1,093 | 23,846 - 31,475 | 26,242 |
| 588-637µm | 637-686µm | 0.185g | 651 | N/A | 1,095 |
| 637-686µm | 955-1,029µm | 1.235g | N/A | N/A | N/A |
Table. 1 - Sclerotia size range and quantity estimate
The quantity counted in the 0.1g samples was then scaled up to estimate the number of sclerotia in the total weight of material in each size group. Additionally for the 0.185g of material which was above this size range but below that of the debris filled larger group the entire amount was counted. The 0.875g of small material and 1.235g of large material was not included.
Based on this the estimated quantity of sclerotia in the remaining 8.22g of material was 110,000 - 140,000 or 123,400 if the average amounts are used. This does not represent a full accounting of the sclerotia produced in this substrate jar due to those lost to the debris and the material previously removed from this jar. The substrate material that was discarded after sieving weighed approximately 17g when dry so it would appear that around a third of the dry weight of the substrate was sclerotia. However it is not yet known what the residual moisture content of the sclerotia is.
Whilst these are only approximations the figures do help reinforce what is seen with casual observation of cultures - the sclerotia are produced in such high numbers that distribution via sclerotia would seem likely.
2.1 Characteristics
The sclerotia of Leucocoprinus cretaceus have a few traits which frustrate attempts to study them: they are very hard, grow in dense clusters, have a tendency to cling to surfaces and are extremely hydrophobic. Due to these traits freshly collected sclerotia will stick to the side of forceps or a needle when attempts are made to isolate them, or move them around on a slide. Dissecting them with a scalpel is not easy and they may just end up stuck to the side of the blade or being pushed aside when attempts are made to cut them. It feels like a grain of sand beneath the scalpel blade and they are prone to being forcefully flung aside when attempts are made to cut them much as a grain of sand would. It is interesting to note that Mattirolo made the exact same comparison to a grain of sand in the Leucocoprinus sclerotia he studied and also used the term 'mycelial envelope' or 'mycelial casing'.[1]
 |
 |
|---|---|
| Fig. 4 - Leucocoprinus cretaceus sclerotia crush mount | Fig. 5 - Leucocoprinus birnbaumii sclerotia crush mount |
The greatest challenge in studying the freshly matured sclerotia is this hardness. When immature and still enveloped in web like mycelium the surface of the sclerotia in L. cretaceus is yellowish and does not pose significant resistance to cutting or crushing. However they harden as they mature such that mounting them on a slide requires deliberate force and the act of crush mounting sclerotia to observe them results in randomly splitting them open as they flatten out (fig. 4). Sectioning them into thin enough slivers to mount without crushing does not seem practical as whilst one cut is possible a second parallel cut near to the first would be exceptionally challenging without more delicate equipment. The sclerotia of L. birnbaumii (fig. 5) with their yellow colouration and mycelial coating look more like the immature sclerotia in L. cretaceus. They exhibit a similar hardness that requires force to cut or crush but appear more prone to squashing and flattening rather than splitting, as such it is possible that attempts to crush mount them can result in the cover glass cracking before they do. At present the sclerotia of L. birnbaumii have not been observed to develop a brown or black colouration with age though it is unclear how they change over a more lengthy period.
The sclerotia of L. cretaceus are difficult to crush beneath the cover slip and require a steady pressure be applied in order to flatten the glass. Slowly pushing the tip of a blunt pair of forceps or the handle of a scalpel against the glass was sufficient to crush isolated sclerotium or small clusters, however they are quite resistant to the force and it feels as if the glass could break before it gives way - and did in a number of cases where pressure was applied too rapidly.
 |
 |
|---|---|
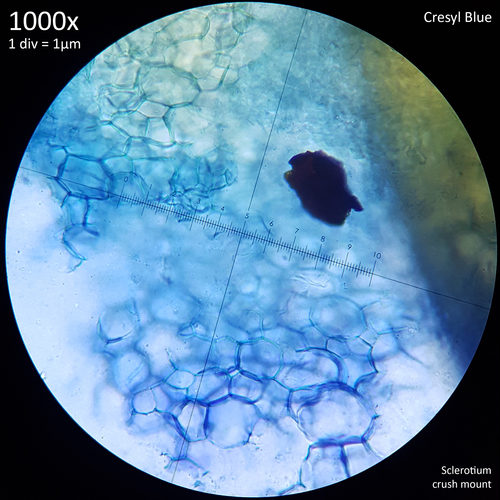
| Fig. 6 - Crush mount in distilled water | Fig. 7 - Crush mount in Cresyl blue |
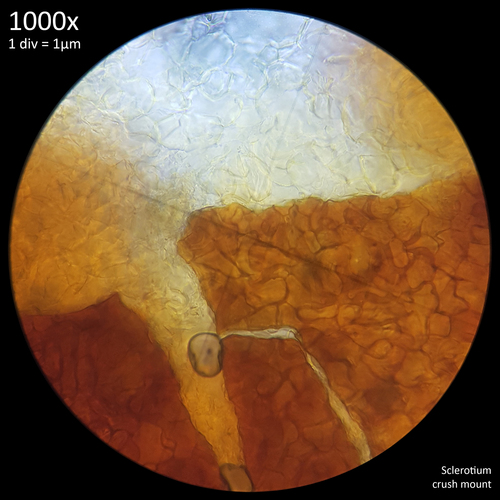
When viewed at 1000x magnification the only distinctive detail that is really observable with the sclerotia of Leucocoprinus cretaceus is the tight mass of hyphae giving the surface (fig. 6) and interior flesh (fig. 7) a highly cellular pattern. The terms pseudoparenchyma and prosenchyma have been used in previous documentation of sclerotia in other species to describe these traits, though this differentiation seems best conveyed simply by the photos as there is some variation in the appearance of the surface and interior between the sclerotia of different species.
The interior flesh which spills out when crushed is hyaline or tinted yellow when amassed and the polygonal cellular texture becomes easier to visualise when stained. The exterior surface appears brown or orangy brown and differs in the texture with it appearing to have smoother, less uniform shapes as opposed to the rather geometric appearance of the internal flesh. Crushed sclerotia do not appear to split in any uniform fashion along seams or along the lines in this pattern.
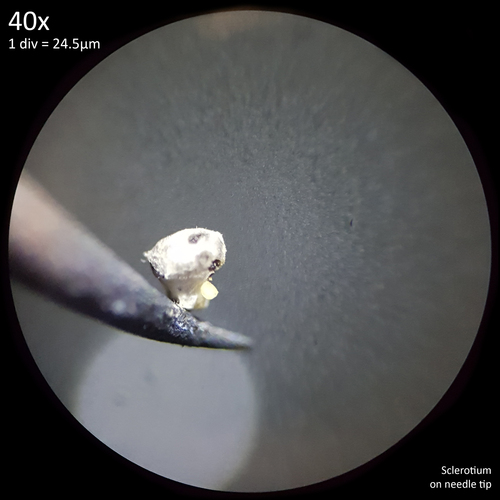
Before crush mounting, whole sclerotia occlude light quite effectively requiring top illumination to visualise them which sometimes results in a similar brown shade to the exterior surface being apparent, though they often appear much darker brown to black. The interior flesh colour can also be seen as distinct from the exterior surface when dissected, though this process is problematic as a result of the size, hardness and tendency to stick to surfaces. This does mean however that individual sclerotium or clusters can be easily picked up just by touching a needle or blade to it with them readily adhering to the surface and not easily dislodging (fig. 8). When mature sclerotia are left to dry they become slightly easier to cut and less prone to sticking to tools but more prone to rolling away instead.
When harvesting mushrooms from jars loose sclerotia will often end up strewn around the work surface and must be collected after or else they will roll and blow around. On a smooth surface like paper they can be blown a significant distance with even a gentle breath but on the slightly dimpled surface of the melamine coated furniture board desk they are surprisingly hard to move. A short sharp breath will propel them a long way but persistent blowing, even relatively forcefully did little to move old, dry sclerotia. Harvesting mushrooms inside a still air box makes for an easy means of containment as the black specks are readily found on the plastic and can be collected just by touching a finger to them, to which they will stick readily.
 |
 |
|---|---|
| Fig. 8 - Sclerotium affixed to needle tip | Fig. 9 - Dissection of fresh, mature sclerotia in 10% KOH |
When placed on a microscope slide with a drop of water, immature or freshly mature sclerotia will float and quickly move to the side of the droplet. They are extremely hydrophobic and any attempt to push them back to the centre will result in them moving to the edge of the drop virtually instantly or sticking to the instrument used to manipulate them. This effect is less noticeable with mature sclerotia that have dried out and it appears to be the mycelial coating of younger sclerotia which provides much of the buoyancy and hydrophobic reaction.
Initially dissection of the sclerotia was achieved by suspending them in a drop of 10% KOH, which appeared to defeat the hydrophobic properties somewhat though it's unclear to what extent it may have saturated or softened the tissue as no perceptible change was noted to either colour or form (fig. 9). In the sclerotia of L. birnbaumii this application resulted in a yellow pigment, perhaps from the mycelium, diffusing into the medium but with L. cretaceus nothing was notable.
Dissection was achieved several more times after the application of ethanol, methanol or 70% isopropyl alcohol although this was performed on older sclerotia which generally appear slightly easier to cut than the freshly matured ones. Isopropyl alcohol resulted in the most marked change in colour (fig. 3) and did appear to soften the structures such that they could be punctured easily with the tip of a needle. It seems probable that isopropyl alcohol is effective at sterilising the sclerotia though it is unclear if other chemicals had any real effect. The application of ethanol or methanol appeared to darken the surface immediately such that it looked dark black but similar was noticed simply by applying distilled water and so this apparent colour change could have just been due to immersion in liquid. After drying, the sclerotia treated with 99.85% methanol looked more matte and perhaps a little more brownish red but were not distinctly different to before. 36.2% hydrochloric acid resulted in a similar blackening to the surface though no obvious corrosion was noted with a brief exposure.
Dissection was easiest to accomplish when sclerotia were viewed at 40X magnification with top illumination either on a slide in liquid to prevent them being flung aside and lost or in a small Petri dish to catch them. This task is more difficult when the sclerotia are freshly matured as they appear harder increasing the chance of them simply shooting out from under the blade. Additionally the mycelial envelope or the remnants of it increase the ability for it to cling to tools.
The interior generally has a yellowish-orange colour but this can appear somewhat muted and grey in some specimens depending on age or with some reagents. Application of Melzer's reagent to the dissected sclerotia of L. cretaceus or L. birnbaumii results in a darkening of the exterior surface with the interior flesh similarly darkening though still retaining a slightly lighter orangy brown colour when excess Melzer's is rinsed away with distilled water. This may suggest that the exterior casing layer has a different composition to the interior, perhaps indicating that the brown or black surface observed in mature specimens of L. cretaceus sclerotia represents the development of a protective coating. Various studies found whilst reviewing the literature note that melanin is found in the brown exterior rind of sclerotia in some Coprinopsis species[2][3] so it seems probable that this also explains the colouration of the sclerotia in L. cretaceus.
 |
 |
|---|---|
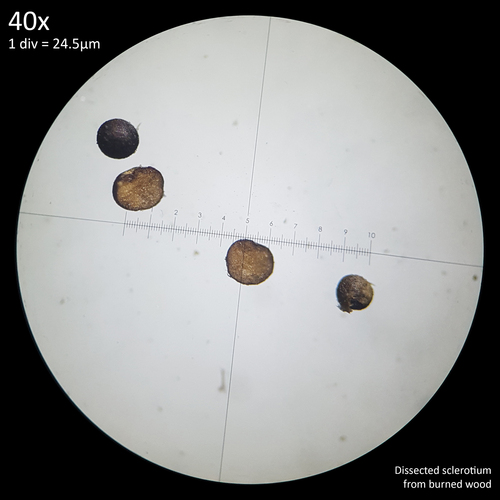
| Fig. 10 - Dissection of dry sclerotium | Fig. 11 - Dissection of burned sclerotium |
Dissecting mature, black sclerotia that had been left to dry for several days (fig. 10) was much easier and did not require the application of any liquid or reagent. Little resistance to the scalpel blade was encountered with the main difficulty being the tendency for them to be flung aside by the pressure.
In order to crudely test their potential resilience to heat the mature sclerotia were exposed to the flame of a mini butane blowtorch resulting in them quickly glowing orange hot and being reduced to ash, after which they could simply be crushed between the fingers resulting in black soot. When placed on the flat side of a scalpel blade and heated from below with the torch they were reduced to white ash before the blade glowed orange hot. However with the lower temperature provided by burning wood they were better able to survive.
Loose sclerotia and some clustered on small pieces of wood substrate (oak fuel pellets) were placed in an unlined steel bottle cap with small pieces of a wooden stirrer (probably Birch) piled on top which was ignited and allowed to burn for around a minute before burning out. The fire was not intense enough to completely destroy the wooden substrate although it was quite blackened and carbonised. Some of the sclerotia were carbonised and crushed easily between the fingers leaving black soot however some appeared to survive and remained hard when rolled between the fingers. Dissection of sclerotia from burned wooden substrate appeared easier with the structures being more brittle resulting in the blade snipping through them without much resistance. Some displayed a more orange interior possibly due to interaction with heat (fig. 11).
Some surviving sclerotia were placed on the back of the scalpel blade which was then heated from below with a piece of burning wooden stirrer. The blade did not glow orange hot but was covered with black soot underneath and was too hot to touch. Some sclerotia shot into the air and were flung away as the blade was heated but others remained on the surface and still retained their form after. Less immediate heating with the blowtorch likewise resulted in sclerotia flinging into the air as the blade heated up. Whether sclerotia exposed to this heat remain viable to grow has not been tested at present as it would be preferable to heat them in a more controlled manner to determine survivability.
2.2 Development of sclerotia and primordia
 |
 |
|---|---|
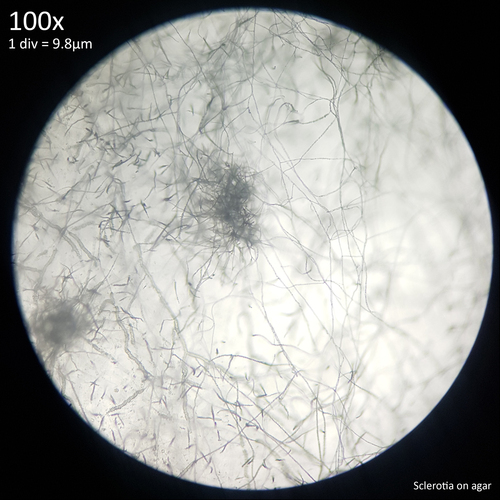
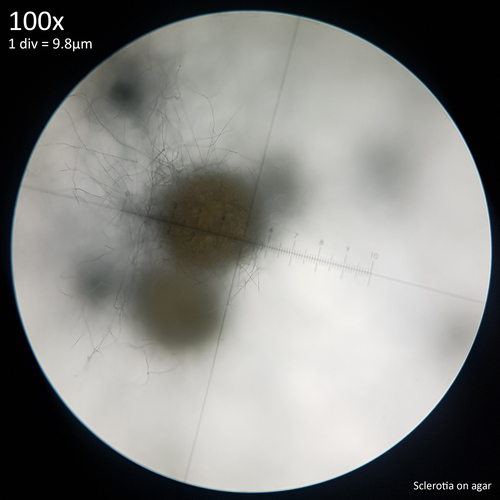
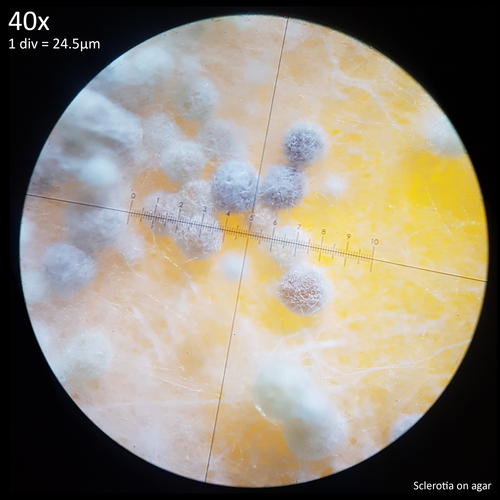
| Fig. 12 - Sclerotia forming from hyphal knots | Fig. 13 - Sclerotia on agar |
The sclerotia form from hyphal knots as can be seen when cultured in agar (fig. 12) and further development results in the formation of a web like envelope of mycelium around the immature sclerotia (fig. 13). Both images here are from the same agar on the same day. At this early stage of the development the sclerotia lack a hard, dark exterior and can be easily dissected with a scalpel revealing pale yellow flesh that appears more gelatinous and lacking in the distinctive features which develop later. The sclerotia of L. cretaceus, L. cepistipes and L. birnbaumii all appear similar at this level of maturity. In sclerotia grown on agar abundant crystal formations are notable on the hyphal tips and crystals can be found inside developing sclerotia when they are dissected or crushed.
These sclerotia were cultured on malt extract agar (1g agar powder, 1g light malt extract powder, 50ml water) which had been sterilised via the no pour agar method in a sealed polypropylene container with a PTFE filter disc. The agar was inoculated from spores on 26/05/23 and left at room temperature, which may have been suboptimal as the mycelium appeared slow growing and when it was harvested one month later on 27/06/23 the plate was only approximately half colonised.
 |
 |
|---|---|
| Fig. 14 - Sclerotia on agar with white mycelium envelope | Fig. 15 - Maturing sclerotia with dark surfaces showing |
As the sclerotia develop further the white mycelial envelope (fig. 14) peels away to reveal the hard, dark surface of the sclerotia (fig. 15). Initially appearing yellow orange and remaining so on the interior, the exterior develops to brown or black with a roughly textured surface that shows a slightly iridescent effect when illuminated with a white LED. When left for longer the sclerotia ultimately become entirely free from the mycelium and to the naked eye appear as tiny black seeds scattered amongst the substrate. At this stage of development such structures could be almost imperceptible amongst soil or easily mistaken for insect frass or debris on wood. Without culturing them on a lighter coloured substrate such as rice or wheat bran in a sealed container they may not be so obvious.
Sclerotia formation was not observed on this agar culture until after the container was opened to harvest colonised agar for propagation. After opening the container Trichoderma contamination developed in an otherwise uncolonised part of the agar and sclerotia growth appeared to begin all over the plate only after the L. cretaceus mycelium made contact with this patch of Trichoderma. This was first observed on 02/07/23, five days after harvesting the agar. At present it is unclear if the contamination prompted sclerotia growth, if the growth was prompted by damaging the mycelium during harvesting, the change in humidity, oxygen or CO2 concentration from opening the container or if it was simply coincidental. It may require a controlled experiment in which contamination is deliberately introduced in order to explore this however there is some reason to think that contamination may have been a factor.
This has also been observed in L. birnbaumii when sections of stipe from a bag of potting soil were placed on agar. Sclerotia growth developed all over and around the stipe section in one plate in which Trichoderma grew from the soil. Whereas in another plate that showed no signs of Trichoderma contamination sclerotia were not observed to develop until much later when the L. birnbaumii mycelium had colonised the plate almost fully. Trichoderma is presumably still present in the non-sterile soil in which this sample was acquired from however a culture of L. birnbaumii continues to grow in this jar with abundant sclerotia and no noticeable mold.
It appears as if the sclerotia may provide some protection against contaminants and if this is the case then contamination prompting earlier sclerotia growth could function as a survival mechanism. In a 500ml mason jar of sterilised wheat bran and wood inoculated with L. cretaceus from agar but contaminated with Trichoderma during inoculation the sclerotia appeared quite resilient to the mold. The substrate was 70g of willow pieces without bark, 10g wheat bran, 1g gypsum and 140ml water sterilised at 15 PSI for 1.5 hours with fresh air exchange faciliated via a 0.3µm PTFE filter disc covering a 7mm hole in the lid. The Trichoderma growth began on the opposite side of the jar to the L. cretaceus inoculation point but quickly swept through the substrate and overtook the leading edges of the L. cretaceus mycelium until more than half the jar was filled with the mold. As it did so however it left the sclerotia behind which remained as white dots scattered amongst the green sporulating contaminant. Even weeks after the Trichoderma had taken over most of the jar the sclerotia remained as white specks surrounded by green. The sclerotia that were surrounded by mold appeared to remain encased in the white mycelial envelope without maturing to black for longer than usual. Where sclerotia were densely clustered the growth of the Trichoderma slowed down or stopped as if unable to spread beyond the barrier created by the agglomerated sclerotia (fig. 16a). The mold appeared unable to colonise the sclerotia and growth of the L. cretaceus mycelium ultimately seemed to be resurgent and was able to recolonise some contaminated areas with new growth appearing around the sclerotia.
 |
 |
|---|---|
| Fig. 16a - Trichoderma contamination amongst L. cretaceus sclerotia | Fig. 16b - Mature L. cretaceus sclerotia after surviving contamination |
This jar was left at room temperature and when checked around two months later (fig. 16b) almost no sign of the Trichoderma was found with only a couple green specks observable on the aborted, dried out mushrooms which had not formed fully likely due to low temperatures. The interior walls of the jar were covered with the mature L. cretaceus sclerotia which gave it a black/grey mold like appearance as is commonly seen in L. cretaceus substrate but the Trichoderma appeared to have lost the competition. After leaving this jar for a further three months no sign of the Trichoderma reappearing was seen. It seemed as if the sclerotia served to provide thousands of inoculation points for the L. cretaceus culture to recover from after the Trichoderma contamination. This has been observed multiple times with jars that were destined for the compost bin due to contamination appearing to only contain L. cretaceus when later checked. Fruiting bodies of L. cretaceus have also been observed to develop in jars that are fully colonised by green and black mold but where some small patches of sclerotia have managed to carve out space.
More controlled experiments will be required to explore this further and determine which kinds of mold L. cretaceus is able to survive as casual observation would suggest that it is more vulnerable to Chaetomium. However if the sclerotia do serve to help the fungus survive competition from microorganisms like fungi and bacteria then it would also seem logical for their growth to be encouraged by their presence. If the sclerotia of Leucocoprinus species provide some resilience to Trichoderma then this could also be a factor in their survival in potting soil and compost.
When Trichoderma contamination occurred in older cultures which had already fruited several times the mature black sclerotia likewise appeared to remain untouched by the mold with little black specks visible amongst the green. The fruiting bodies and old myceliated substrate easily fell prey to the mold however. The mature sclerotia also appeared to be able to survive infestation by mites (likely Tyrophagus putrescentiae) as whilst these pests ultimately bred abundantly, consumed the mycelium and mushrooms and destroyed the culture the same as they do for other species, the sclerotia appeared to remain untouched. It is possible that by virtue of exuding liquid during maturation the old sclerotia become too dry and hard to be consumed by mold or mycophagous pests.
A study on sclerotia formation in Coprinellus congregatus found that the presence of low levels of bacteria in quantities difficult to detect appeared to induce sclerotia growth.[4] As such it would seem to be hard to categorically state that sclerotia growth has also been observed in uncontaminated cultures of Leucocoprinus cretaceus. At best I can say that it has also been observed abundantly in cultures that do not appear to be contaminated with every agar plate or substrate developing sclerotia. The number of sclerotia which develop and the time they take to grow appears linked to the nutritional content of the substrate.
Cultures on water agar (1g agar, 50ml water) will also produce sclerotia though they are far fewer in number with only five or six specimens beginning to develop months after inoculating. By comparison cultures on malt extract agar were crowded with sclerotia within weeks which in some places were so numerous as to form piles of mature, black sclerotia several millimetres high.
As the sclerotia mature on agar a crystal clear exudation forms on the surface of the sclerotia producing a glistening appearance. This persists for several days before discolouring yellow and ultimately evaporating or being reabsorbed into the agar, leaving the dry black sclerotia behind. This trait was noted by Mattirolo in his attempts to cultivate the Leucocoprinus sclerotia he studied however he noted that after the exudation discoloured, contamination from mold destroyed his cultures.[1] The exudation however doesn't appear to be related to contamination and is merely a part of the maturation process in which the sclerotia dry out.
Exudation is reported in many of the studies on sclerotia in many species and is also covered in H. J. Willetts' comprehensive 1971 study of sclerotia which primarily focuses on plant pathogen species though does contain much information that appears universal to sclerotia. Specifically it is noted that sclerotia exude water after beginning to increase in size resulting in the sclerotia losing water content, which may increase their survivability. It is said that everyone who has studied the formation of sclerotia will have observed the moisture droplets on their outer surface.[5] Indeed this was very visible and hard to miss with the sclerotia of L. cretaceus or L. cepistipes when cultured on agar.
 |
 |
|---|---|
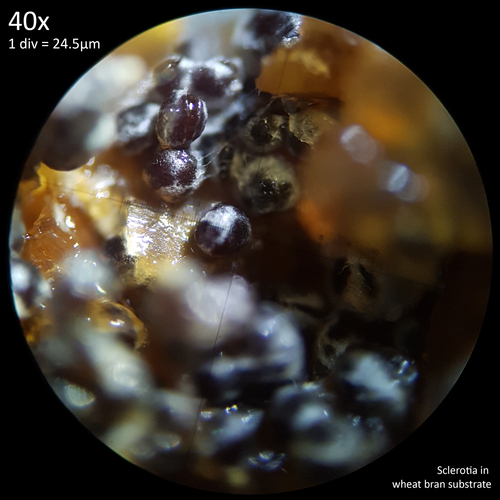
| Fig. 17 - L. cepistipes sclerotia with exudation | Fig. 18 - L. cretaceus sclerotia on wheat bran |
The sclerotia of L. cepistipes are smaller than those of L. cretaceus but ultimately quite similar with their dark colouration at maturity. Observations of the exudation from this species (fig. 17) are interesting in that the droplets formed by the exudates can appear much larger than the sclerotia themselves. With either species these droplets are not readily apparent when sclerotia are grown in substrate. Even when a nutritionally rich substrate like wheat bran (fig. 18) produces abundant sclerotia growth exudation does not seem to be observable. It is not clear if this is the result of exudation being more quickly absorbed into the substrate, evaporating faster in the larger container or being formed slower due to the less saturated substrate.
Even without a microscope the sclerotia are visible with the naked eye, starting as tiny, white, woolly looking specks on the substrate which were abundantly obvious in culture prior to any further investigation. In nature or in a plant pot however these could be easily missed or dismissed as mycelium unless the observer was actively looking for them. When the sclerotia were produced in jars of soil substrate they were readily seen against the side of the clear container whilst surrounded by the mycelial envelope but were not so apparent on the surface once mushroom production began. They were routinely collected along with harvested mushrooms but were not easily isolated from the substrate and would be hard to find when mature and black. Cultivation on brown rice proved optimal for study and collection.
 |
 |
 |
 |
|---|---|---|---|
| Fig. 19a - 29/07 - Sclerotia collected | Fig. 19b - 02/08 - First primordia observed | Fig. 19c - 04/08 - Mushrooms becoming distinctive | Fig. 19d - 08/08 - Some aborted pins shrivelling |
Sclerotia growth was first observed most abundantly in a jar comprised mainly of brown rice with added nutrients where widespread growth was visible with the naked eye (fig. 19a). This was inoculated from the donor agar (fig. 14) on 27/06/23 and small numbers of sclerotia were first observed not long after finding them on the agar. The substrate for this jar was 75g brown rice, 0.75g yeast extract, 0.75g soluble starch (potato), 0.75g gypsum, 20g hardwood fuel pellets (oak), 105ml water in a tall, 500ml mason jar, filled about half full with a PTFE filter disc on the lid. This had been prepared and sterilised on 23/04/23 for another experiment but went unused and so the substrate appeared dry and likely was underhydrated to begin with. The wood on the surface was very dry and little growth was observed on it however the rice became covered in sclerotia, which grew continuously as the mycelium slowly spread down the jar. Note that none of the extra ingredients added are necessary and a substrate simply comprised of 40% brown rice to 60% water by weight produces abundant sclerotia and fruiting bodies.
Sclerotia were harvested from this jar for study on 29/07/23 and within four days on 02/08/23 primordia began growing around the area of the substrate that was disturbed (fig. 19b). This growth was initially only observed at the site where the substrate had been dug into with forceps to collect myceliated rice and sclerotia but pinning spread gradually out from this area. It appeared as if disturbing the mycelium prompted fruiting however this site was where the most developed sclerotia were and likely was the site of inoculation from the agar. With the densely clustered growth of both the sclerotia and primordia it could appear as if the sclerotia were themselves growing into the mushrooms. However dissection of immature pins revealed solid white mushroom flesh with no traces of material from sclerotia. With larger mushrooms sclerotia were often stuck to the exterior surface and sometimes slightly embedded such that they had to be pried out rather than casually brushed off. However no sclerotia were found inside any fruiting bodies and it appeared as if sclerotia were mostly pushed out of the way by developing fruiting bodies rather than subsumed into them.
As the mushrooms became larger and more distinctive in their form (fig. 19c) it became harder to observe the sclerotia amongst them. The largest mushroom that was initially produced filled the space where substrate was harvested from but it formed strangely and stopped growing. At this time the jar was just sat at room temperature and was only around 20C. This appeared less than optimal for growth and so the jar was placed on a heated plant tray on 06/08/23 which brought the base of the jar up to around 26-28C but the top still felt cool to the touch. The large mushroom however ultimately aborted, as did some smaller ones around it. These became shrivelled, looking almost deflated and gradually developed a yellow tinge (fig. 19d).
The jar was elevated further from the heated tray to prevent overheating the substrate and an LED plant grow light was placed in front of the jar, putting out a reasonable amount of heat and warming the glass. With the combination of the heat pad and light placed on a shelf covered by a plastic sheet on the front and cardboard on the sides, the ambient temperature around the jar rose to 26-28C. Mushroom development appeared to speed up with the increase in temperature and many large specimens formed on the surface.
 |
 |
 |
 |
|---|---|---|---|
| Fig. 19e - 09/08 - Mycelium obscuring sclerotia | Fig. 19f - 09/08 - Dense, caespitose growth | Fig. 19g - 10/08 - Rapid growth in warmth | Fig. 19h - 11/08 - Mature specimen harvested |
As the growth increased so did the spread of the mycelium with white, whispy mycelium beginning to obscure the sclerotia (fig. 19e) and the dense, caespitose growth of the mushrooms obscuring the substrate and the sclerotia upon it (fig. 19f). The heat appeared to result in numerous mushrooms developing well (fig. 19g) however after a mature specimen was harvested from this jar (fig. 19h) the remaining pins aborted and began to shrivel. Harvesting the mature mushroom resulted in the interior of the glass and the substrate becoming coated with powdery scales from the cap and stipe which further obscured the sclerotia.
As a result it is not surprising that sclerotia go unnoticed in this species. The first point at which people are likely to notice this species growing in their plant pot, greenhouse or planter is when the mushrooms appear and by that point the sclerotia are already too hard to see. Scales from L. cretaceus readily detach in rain or when handled with white specks coating the area around them and so any immature sclerotia that are visible could easily be mistaken for scales or primordia. Whereas with Leucocoprinus birnbaumii the sclerotia are often observed before any mushrooms appear since they are distinctly noticeable with their persistent yellow colouration. The mature black sclerotia of L. cretaceus can often be found on the base of the mushrooms but if they are grown on soil these are not especially evident amongst the brown/black particulate matter of the soil itself. This may explain why Mattirolo stated that his Lepiota cretacea did not produce sclerotia as his observations were made on specimens collected from hothouses and he was never able to successfully culture Leucocoprinus species.[1]
2.3 Propagation
The sclerotia make for an exceptionally easy method of inoculating new substrates as when colonised rice grains are harvested and put to new substrates some sclerotia will invariably detach and end up strewn around the surface. This results in multiple inoculation points occurring from even small amounts of rice and in cases where single sclerotium have fallen into a gap between the substrate and the side of the jar growth can be observed quite rapidly.
When jars of rice are sufficiently colonised they become filled with so many sclerotia in such dense clusters that it is not always apparent if taking a pinch of the substrate with forceps has resulted in harvesting any rice or if only sclerotia and mycelium are present. In practice it does not seem to matter as growth from clusters of sclerotia can be just as effective as from colonised grains of rice.
For the purpose of these experiments it proved optimal to simply take a pinch out of a colonised rice jar and tap it on the side of the new jar to propagate. Numerous jars could be inoculated in this manner from a single donor jar using only a few grains per jar. After inoculation with a pea sized clump of mycelium and sclerotia if the jar is shaken gently side to side (without disturbing the substrate) these clumps can be broken up resulting in sclerotia being scattered all across the surface. The effect of this is reminiscent to using popcorn spawn to inoculate bulk substrate resulting in many inoculation points for rapid colonisation.
This method of tapping the forceps on the side of the jar or shaking the jar afterwards also resulted in many isolated sclerotia being stuck to the inside walls of the polypropylene or glass jars. Even when they were not in contact with or close to the substrate these sclerotia were able to grow and mycelium was readily observed spreading from the little black dots on the glass. This growth was presumably the result of using the stored nutrients in the sclerotium to feed the mycelium and was likely triggered by the high humidity in the jars, which may have provided a water source via condensation. Growth on the glass or plastic did not appear to spread beyond a couple centimetres in diameter unless contact was made with a nutrient source, in which case new sclerotia formation occurred on the glass in a ring around the original and then clustered on the nutrient source.
Even if attempts are made to inoculate a substrate in a controlled manner from a single grain placed in one location it is prone to multiple growth points from loose sclerotia as they are so easily scattered. The growth from single sclerotium often appears to be just as healthy and rapid as from a much larger clump of colonised rice. However it is not always clear if the growth is from the sclerotium itself or from residual mycelium stuck on the surface.
 |
 |
|---|---|
| Fig. 20 - Mycelium growing from sclerotium on agar | Fig. 21 - Sclerotium dissected and crushed |
When a single sclerotium harvested from a soil substrate was placed on malt extract agar mycelial growth was first observed emerging in a neat circle around it six days later, though it was only being intermittently checked. This sclerotium appeared fully mature, black and without noticeable mycelium remaining on the surface so this growth appears to be from the sclerotium itself though more controlled experiments with surface sterilisation may be necessary. The agar container was opened 15 days after inoculation in order to harvest it for study (fig. 20). The exterior of the sclerotium was encrusted in mycelium and agar which was easily removed by manipulating it with a scalpel under 40x magnification. When removed the exterior surface appeared brownish black without discernible difference to the appearance when first collected. It remained hard and took force to dissect with the scalpel. After dissection the two halves were crush mounted in distilled water (fig. 21), which also took force. When compared against a mature black sclerotium that had been left to dry no discernible difference was noted, besides the presence of crystals, which may simply have come from the agar as examining an uncolonised section of the agar revealed similar crystalline structures.
When a jar of brown rice (60g brown rice, 90ml water) was inoculated from sclerotia, mycelium spread out in a circle around isolated sclerotium that had fallen between the glass and the substrate. When the mycelium reached approximately 3-4cm in diameter new sclerotia began developing all around the edges forming a broad ring of scattered sclerotia surrounding the mycelial patch. The appearance was reminiscent of the mushroom distribution in a fairy ring forming species like Marasmius oreades though this effect was short lived as the entirety of the visible substrate soon became colonised and covered in large quantities of sclerotia. In jars containing a mix of wheat bran and wood or brown rice and wood the sclerotia formed primarily on the wheat bran/rice rather than the wood. Mushrooms harvested from wood had few to no sclerotia stuck to the base of the stipe as opposed to mushrooms harvested from rice or potting soil which were covered in sclerotia at the base. The addition of a layer of wood to the top of a rice or soil substrate was effective at producing mushrooms without sclerotia around the base.
In all cases in which mushrooms were produced they only developed after sclerotia were widespread and mushroom production was so readily achieved as to occur in almost all substrates, even those using just small amounts of substrate intended only to study sclerotia growth. Whilst mushrooms would sometimes be seen to grow abnormally or abort and shrivel up if room temperature became lower sclerotia appeared to develop abundantly regardless.
In cultures on wheat bran (10g wheat bran, 20ml water) the mycelium spread across the surface in rippling waves with sclerotia forming in the second wave behind the first, often elevated well above the substrate in the whispy masses of aerial mycelium. When sclerotia were harvested from a substrate of wheat bran and hops (20g wheat bran, 0.4g hops, 50ml water) which had produced mature mushrooms, the substrate appeared like black soil in places owing to the vast numbers of mature sclerotia that had amassed (fig. 18). Digging down into this substrate with forceps revealed that this black mass of sclerotia continued for at least a few centimetres below the surface. The substrate was not especially dense due to the vast numbers of sclerotia which seemed to make up a significant portion of the substrate creating a consistency somewhat like moist, coarse sand. Clumps consisting of dozens or hundreds of sclerotia were easily picked up with forceps to propagate to new substrates.
Growth of sclerotia was also noticeable when jars of sterilised potting soil (verve multipurpose peat-free compost 50L from B&Q) were inoculated with glucose liquid culture, from agar or from a few grains of colonised rice with sclerotia. Greater numbers of sclerotia were produced in jars with some barley straw or wheat bran mixed in to the soil to provide additional nutrition and the addition of various leaves and plant debris to simulate leaf mulch likewise appeared beneficial. Sparse, slower growth of sclerotia was observed when pieces of wood (willow) were added to the soil and in substrates primarily comprised of wood sclerotia growth appeared much poorer than in those primarily comprised of soil. Whilst Leucocoprinus cretaceus is often observed growing from wood in nature it appears as if wood is less optimal for sclerotia growth.
Development was quite abundant in jars containing only unamended potting soil with a ratio of 100g soil to 60ml water resulting in quick mycelial growth followed by widespread sclerotia development and ultimately fruiting bodies. The addition of coco coir to the substrate (Haxnicks growlite premium coir mix containing added nutrients, dried seaweed and hormones) proved even better with faster growth, greater numbers of sclerotia and more rapid development of fruiting bodies. A substrate comprised of 15g coir, 50g soil and 110ml water resulted in improved growth over the soil alone. In both of these jars sclerotia were observed 10 days after inoculating. It would seem probable that the increased growth is mainly the result of the coir enabling a higher volume of water without oversaturating the substrate though Leucocoprinus species may also be well suited to growing on coconut debris. Some Leucocoprinus species were described from waste material at coconut plantations and observations from terrariums or vivariums containing only coir are common.
The added nutrient content of this coir is not known although it seems low as plants started in it grew very poorly or failed entirely and it has proven effectively useless for this purpose. It likewise proved unsuitable for bulk mushroom substrates as contaminating mold grew far better than the desired mushroom cultures, which may be the result of the added seaweed.
In a jar containing 30g coir and 110ml water almost no growth from L. cretaceus was initially observed at all. Even whilst every other substrate test in this batch was well colonised this jar remained the only one without noticeable growth with at most a tiny patch of mycelium at the inoculation site. 46 days after inoculation maturing white sclerotia were observed in a reasonable quantity in some locations though were sparse in the substrate as a whole. They were primarily found towards the bottom of the jar on the side which had been facing the heat pad and light where condensation had collected. Growth may have been slow due to the lack of a nutritious substrate or inadequate hydration of the coir as in jars of underhydrated soil sclerotia are seen to develop much later.
Mycelial growth from sclerotia can also occur in water even without nutrition being added. Sclerotia were dropped into non-sterile tap water, rainwater or distilled water with some individual mature, black sclerotium, some in small clusters, others in larger groups interwoven with mycelium or some tiny primordia and some still attached to pieces of rice or wood substrate. All initially floated besides those weighed down by rice. Stirring the water did not result in breaking up the clusters or sinking them, though some isolated, black sclerotium sank. The clusters that retained some mycelium were strongly attracted to the wooden stirrer or scalpel when it was placed in the water approximately 1cm away and quickly moved towards it, accelerating as they got closer. Upon touching the surface they stuck to it, perhaps as a result of surface tension and remained attached when it was lifted from the water. In some cases they stuck so firmly to the scalpel blade or wooden stirrer that they had to be scraped off as dipping them back in the water did not remove them. The mechanism of attraction and then adhesion was so reliable that it could be repeated multiple times without failing and when left undisturbed naturally happened with them sticking to the side of the polypropylene container.
This effect however was short lived for most of the sclerotia. When the containers were examined again after five hours most of the sclerotia had sunk, including some patches with mycelium that had so easily stuck to the tools before but now lacked this trait. Isolated black sclerotium that lacked a coating of mycelium had all sunk and when more were added from material that had been left out to dry after harvesting they either sank immediately or floated but were easily sunk with a light touch. It therefore appears that the hydrophobic reaction and the buoyancy is not the innate product of the sclerotia but rather the mycelial envelope that coats them before they are fully mature. A cluster that contained two or three sclerotia attached to a very tiny and immature primordium remained floating and would not sink, though these did not so readily attract and cling to surfaces. Another cluster with a larger primordium became saturated and easily sank before the others. The sclerotia do have a tendency to become clustered around the base of the mushrooms, not swallowed up inside the flesh but stuck firmly enough to the exterior or tangled amongst the floccose coating so as not to be removed easily. When the mushrooms are very small this appeared to provide the sclerotia with something akin to a floatation device.
4 days after introducing the sclerotia to distilled water most had sunk but a cluster of a few sclerotia that appeared to be attached to a tiny, very immature primordium were still floating in the water and white mycelium was visible spreading from the cluster and growing across the surface. One month later these were still afloat and the mycelium still appeared to be alive and growing, though had not spread substantially likely owing to the lack of nutrients in the water. Similar was observed in tap water where a cluster of approximately 50 sclerotia with mycelium remained floating and started growing new mycelium and in rain water where a cluster of 5 or 6 sclerotia as well as some isolated ones remained floating and growing. Besides these floating patches present in each container a month later, most sclerotia had sunk but some submerged mycelial growth was also observed. Some of the sunken sclerotia appeared to have a deep reddish purple colour when illuminated and there was a fuzzy white veil around them.
This basic experiment as well as the observed traits of hardness, hydrophobia and sticking to anything that touches them may reveal some important clues as to the function of these structures in nature.
3. Evolutionary advantages of sclerotia in Leucocoprinus species?
Observations of sclerotia in Leucocoprinus species are generally limited to L. birnbaumii in plant pots or from bags of compost with the sclerotia being much harder to see in observations of L. cretaceus or L. cepistipes such that there are fewer observations of them. Sclerotia are not commonly mentioned in the literature and their presence or absence does not appear to have been documented for most described species. Mattirolo's study appears to be the most comprehensive and he only documented sclerotia in a yellow species that he described as Lepiota flos-sulphuris[1] which appears to be Leucocoprinus birnbaumii and a pale species illustrated with a brown centre which he described as Lepiota incerta.[1] This may possibly be Leucocoprinus ianthinus or a similar species since L. lilacinogranulosus is considered a synonym though does differ in its description.
Due to the lack of documentation it is difficult to assess what function the sclerotia may serve in nature since it is not yet known which other species produce sclerotia, what the properties of those sclerotia are and how these may relate to the habitat of the species. The properties of the sclerotia of Leucocoprinus cretaceus and the observations of its habitat however make for some intriguing possibilities with which to hypothesise on.
Sclerotia being produced quickly and in vast numbers before any fruiting bodies appear, even when conditions are optimal for them, suggests that whatever role the sclerotia play it must be an important part in the fungal life cycle. The key question to answer is whether the sclerotia simply serve to survive and persist or actively spread.
3.1 Surviving extremes?
Willetts stated that 'It is generally accepted that sclerotia are able to survive conditions that are too severe for ordinary vegetative hyphae and spores' noting that sclerotia were a 'very formidable means of perpetuating a fungal species'. The paper also notes that the large size of sclerotia tends to confine them to the location where they develop rather than being spread via wind and air currents like spores, though the small sclerotia of Leucocoprinus species are not discussed. Sclerotia are said to provide some resistance to extremes of heat and cold but appear better adapted to surviving extremes of cold temperature. In the case of plant pathogen species this enables them to lay dormant in the soil during the cold months in order to survive until the next growing season.[5]
However if Leucocoprinus cretaceus is assumed to be a tropical species that is native to South America, as is typically suggested, then it would seem unlikely for the evolutionary trait of sclerotia production to become so prolific and seemingly prioritised over mushroom production if the only purpose it served was surviving hardship like cold temperatures. The vast quantities of dense, hard sclerotia that are produced before any mushrooms surely requires some significant energy and nutrient usage that could instead go towards producing mushrooms or growing the mycelium out further, faster. Whilst it can be expected that the number of sclerotia produced in sterile, high nutrient cultures is likely greater than the amount produced in natural environments observations in which sclerotia are visible amongst the mycelium still suggest a great abundance.
An assessment of the temperatures during 200 iNaturalist observations of wild fruiting bodies of L. cretaceus was carried out by looking up the temperatures provided by www.timeanddate.com and noting the highest and lowest temperature on the day based on the location given. The lowest temperature was -2°C although with this observation it was possible that the microclimate in the park in which it was found may have resulted in a higher temperature than was recorded elsewhere in the area. The next lowest was 4°C. The average minimum temperature was 21.64°C and the average high temperature was 29.23°C. The lowest high temperature was 14°C, the highest high temperature was 42°C and the highest low temperature was 30°C. The information has been collated in https://www.inaturalist.org/posts/84531-weather-table
Reviewing the observations of L. cretaceus from South and Central America reveals year round growth with mushrooms growing in the wild during every month of the year. Most of these locations do not see significant cold periods with freezing temperatures being rare suggesting that it is unlikely for an evolutionary forcing to exist that requires the production of sclerotia to overwinter. The thick-walled spores of L. cretaceus may already be adequate at surviving the brief periods of cold sometimes seen in these South American countries and with fruiting bodies produced year round fresh spores are likely always present in the environment.
In the temperate regions of Europe and North America observations of L. cretaceus are less common and often confined to plant pots or compost piles during the warmer Summer months. In Texas and Florida L. cretaceus can be observed in Autumn and Winter months however and is not uncommon to find growing outside. If the sclerotia do provide some resistance to the cold, which seems probable, that may be a useful trait in the environments this species has spread to however it would not seem to be of great importance to surviving in its native environment given the temperatures typically found there. Logically if a mutation occurred that resulted in fewer or no sclerotia being produced with mushrooms being prioritised instead then this genetic line could be expected to become dominant given the greater spread via spores this would result in. This may suggest an alternative purpose for the sclerotia that has resulted in the trait becoming dominant.
A purpose which is often suggested for sclerotia in other species is surviving wildfire. This has been hypothesised as the purpose for the sclerotia in Conocybe cyanopus and some grassland Psilocybe species.[6] It is also well noted that Morels have an association with fire and the deep rooted, large sclerotia produced by some of these species do seem ideal for surviving fire and fruiting afterwards to take advantage of the freshly sterilised surface substrate. However the tiny sclerotia produced in vast numbers at or near the surface of the soil as can be seen in L. cretaceus do not seem like a logical adaptation to surviving fire.
Studies of the sclerotia produced by the plant pathogen Sclerotinia sclerotiorum determined that stubble burning in fields was not an effective control method as sclerotia were capable of surviving the fires. The survival rate was dependent on the density of the stubble and the temperatures reached and fell in proportion to the higher temperatures created by denser stubble burns. Interestingly the size of the sclerotia was not determined as affecting the ability to survive stubble fires.[7] However the size range given for the sclerotia in this species is 800-1720 µm[8] and so they are larger than the typical size observed in L. cretaceus.
Whilst the sclerotia of L. cretaceus do show some tolerance to heat it seems probable that this is going to be short lived owing to their diminutive size and lack of significant soil mass to shield them. Some sclerotia may get flung into the air by the heat as is seen when heating them on metal but in a wildfire scenario this does not seem like a reliable method of escape as most would surely just land in burning debris or hot ashes elsewhere. More importantly, rainforests generally are not prone to fire, or at least were not until people started burning them. It therefore seems unlikely that sclerotia production in L. cretaceus is an adaptation to surviving fire given this fact as well as the nature of the sclerotia produced.
It seems probable that sclerotia do serve to aid this species in surviving extremes just as they do in other species - sclerotia appear to be naturally durable structures. However as the extremes of cold and fire are seldom found in this environment and as this species thrives in high temperatures with mushrooms produced year round these do not seem a satisfactory explanation for sclerotia being produced so abundantly. One simple explanation may be drought as these regions have in some cases experienced major droughts during El Niño years.[9] Sclerotia, with their tendency to exude water and dry out seem likely to be a good mechanism for laying dormant during periods of drought and then rapidly regrowing once the rain arrives. It seems highly likely that they would survive such conditions better than hyphae or spores but unlikely that they would all just remain where they formed once the rains return.
Reviewing the observations of L. cretaceus from South and Central America has revealed an environment which seems ideally suited to inoculation via sclerotia if an appropriate distribution method is available. Splitting palm trunks, their exposed brush like roots or climbing vines against trees provide so many nooks and crannies for sclerotia to get stuck amongst and take hold as do the nutritionally rich termite mounds and ant nests.
3.2 Distribution via sclerotia?
Whilst the rainforests are not prone to natural disasters like fire they are prone to something else which could prove equally catastrophic without adaptation: flood. Seasonal flooding is a characteristic of many of the locations in South America in which L. cretaceus is naturally found.[9] The tiny, numerous, highly hydrophobic (when immature) sclerotia found on the surface of the soil could readily be dislodged by flooding or even heavy rainfall. Some amount of these sclerotia, where attached to enough mycelium or immature primordia could remain floating for quite some time and would be prone to clinging on to any debris that floated past like sticks, leaves and clumps of soil. Isolated mature sclerotium would sink, be swept along by the current and may end up buried in the freshly deposited soil or falling into crevices on the bottom of the flood channel. Sclerotia could be readily distributed through the flooded environment with the brush like texture of palm roots catching them and every flooded termite nest providing countless holes for sclerotia to end up in. The properties possessed by the sclerotia seem virtually ideal for distribution in this manner and they may be able to survive and regrow after such upheaval better than the spores.
This principle was tested by introducing clumps of sclerotia to a variety of sterile, submerged substrates including some with various plant material (straw, sticks, stems) placed vertically in a jar half filled with rain water. A few hours after introducing the sclerotia some were found clinging to the sodden stems well above the water whilst others floated on the surface or clustered around stems at the water level. Tilting the jar to raise or lower the water level on one side resulted in floating sclerotia sticking to the plant material and then remaining there, above the water line when the water level fell. Sclerotia remained floating days later with mycelial growth visible on the surface but when this tilting was repeated some clusters remained affixed to the plant material and were not relocated whilst others remained floating but with mycelial strands anchoring them to the debris.
 |
 |
 |
 |
|---|---|---|---|
| Fig. 22a - Jar 1 - 07/11/23 | Fig. 22b - Jar 1 - 27/09/23 | Fig. 22c - Jar 2 - 07/11/23 | Fig. 22d - Jar 2 - 27/09/23 |
Jar 1 (fig. 22a) had a substrate comprised of 1.5g plantain (Plantago) stem, 3g willow wood, without bark and 1g wheat straw cut into lengths to stand upright in the jar above the 260ml of rain water. These jars were inoculated on 18/09/23 from pieces of a wheat bran substrate (fig. 18) that was well colonised and full of sclerotia. Subsequently there were numerous inoculation points as a result of sclerotia coming loose from the substrate as it was placed into the jar. Immature sclerotia and clusters of mycelium floated whilst mature sclerotia sank. 9 days after inoculation mycelial growth was present both on the plant material and floating on the surface of the water (fig. 22b).
Jar 2 (fig. 22c) had a more nutritious substrate comprised of 1.6g plantain (Plantago) seed heads and stem, 1g plantain stem, 1.5g wheat seed heads, 1g wheat straw, 5g willow wood, without bark with the pieces likewise cut to stand upright in the jar of 260ml rain water. This was inoculated at the same time in the same manner. Much greater mycelial growth occurred with more sclerotia produced on the plant material and immature primordia forming however Trichoderma contamination was also present in this jar and so whether the more numerous sclerotia growth was due to the contamination or the more nutritious substrate is not clear. The jars were stored at room temperature which was adequate though probably suboptimal for growth as the control jar of rice substrate developed malformed or aborted primordia consistent with lower temperatures. 9 days after inoculation many floating sclerotia had become stuck to the wheat grain and mycelium was quickly spreading from the base up the seed head (fig. 22d). 80 days after inoculation the mycelium from L. cretaceus appeared healthy on both the water's surface and the plant material with sclerotia at all stages of development still present and some aborted primordia. The floating patches of Trichoderma were not able to spread as significantly as the L. cretaceus.
 |
 |
 |
 |
|---|---|---|---|
| Fig. 22e - Glucose solution - 07/11/23 | Fig. 22f - Yeast extract solution - 07/11/23 | Fig. 22g - Soil solution - 07/11/23 | Fig. 22h - Sclerotia on surface - 07/11/23 |
Jars containing liquid substrates without plant material were inoculated at the same time and in the same manner. Mycelial growth was also observed from floating clusters of sclerotia placed in 14g glucose in 350ml rain water (fig. 22e), 7.5g yeast extract and 12g glucose in 300ml rain water (fig. 22f) and 60g potting soil in 300ml rain water such that the soil was entirely submerged (fig. 22g). It was expected that the yeast extract and glucose solution would perform best as this mix is capable of resulting in significant submerged cultures in Cordyceps militaris and other species probably due to the high nitrogen content however surprisingly the best mycelial growth was noted in the submerged soil jar, despite the only nutrition added to the water being suspended soil sediment.
Growth in the suspended soil jar rapidly outpaced that of the glucose or yeast extract solutions and new sclerotia were formed floating on the surface which were readily seen when exudation began (fig. 22h). The exudation was first observed in the soil jar 15 days after inoculation when the floating mycelial patch was only 2-3 centimetres wide, so production of sclerotia will have started a few days to a week prior to this but they were not clearly apparent until exudation occurred. This is consistent with the control jar of rice in which the top centimetre of the substrate had become crowded with maturing white sclerotia 15 days after inoculation.
No new sclerotia were observed to grow in the glucose or yeast extract. In the glucose jar mycelial growth was also present from the sclerotia which had sunk to the bottom of the jar, each of which became surrounded in a fuzzy orb of mycelium. Similar growth also appeared to be present from the sunken sclerotia in the yeast extract when the jar was illuminated to view them but was not observed in the other jars.
Floating sclerotia growth was also seen when a jar of sterilised submerged soil was inoculated from sclerotia but then left open to the environment via uncovered holes in the lid in a warm grow tent with chilli plants which were heavily infested with numerous fungus gnat species. In this non-sterile environment Trichoderma quickly appeared floating on the surface of the jar and ultimately the liquid became filled with bacteria with some microbial mat production and a smell like a bog. Within the first weeks however Leucocoprinus cretaceus was able to grow a floating patch of mycelium a few centimetres wide which produced many new sclerotia before the culture succumbed and diminished.
Preliminary tests with non-sterile environments have also been conducted by placing soil that has already been colonised in a large, sealed container without air holes and filling it with rain water that had been sat outside in an open barrel. The bulk of the soil substrate was submerged so as to crudely simulate a flooded environment with some pieces floating and forming a layer on the surface. This initially resulted in very good surface growth with floating patches of mycelium and sclerotia being seen to rapidly produce new growth across the surface of the debris filled water. This growth appeared to be able to persist for some months but ultimately fell prey to bacteria with slimy microbial mats and foam replacing the previously healthy looking growth. When the flooding test was repeated with the addition of a freshly unearthed sweet potato root crown or sweet potato stems sitting in and above the water in a jar with filtered air holes this material quickly became colonised but as it became oversaturated and rotted growth on the plant material subsided, though the floating mat remained thick.
No new growth is seen below the water line on submerged plant material or soil in any of these experiments and in oversaturated, but not submerged, soil jars growth is limited to the top layer. In a naturally flooded environment then it could be expected that the mycelium of Leucocoprinus cretaceus which remains in the soil beneath the water may suffer, cease growing and fall prey to microorganisms better suited to anaerobic or submerged conditions. The sclerotia that remain in the soil might help serve as a survival mechanism to recover after the water recedes whilst some clusters of mycelium and sclerotia from the surface could be expected to float away. The mycelium is quite fragmentary due to the high numbers of sclerotia so flooding may result in many separate pieces being carried away. It would seem unlikely for sufficient floating growth to occur and persist for long outside of a sterile environment though potentially new sclerotia could form in floating patches if they are able to survive for a couple weeks. In such a flooded environment it seems more likely that these clusters would become stuck to plants, trees and debris that sit above the water line. This may result in rapid growth which anchors the clusters to the debris and quickly colonises the material to escape the flood water. The rapidity with which new sclerotia can be formed and their tendency to form in aerial mycelium on saturated plant material would help ensure survival if further flooding occurs and waters rise.
It will be necessary to further test this idea in non-sterile substrates with various plant material and compare results against similar saprotrophic species which do not produce sclerotia. Comparing growth in this environment against inoculation from spores may also help determine whether sclerotia provide a sufficient advantage at surviving and spreading through a flooded environment.
In a region that is as prone to flooding as South America the ability to effectively spread via flood waters and then quickly colonise the disturbed ground and amassed debris could be highly beneficial. In a flooded environment with gradual drainage this mechanism could result in sclerotia being distributed all through piles of debris as the water level falls resulting in inoculation from multiple points. This may provide an explanation for many of the observations in which the mushrooms are observed growing from naturally occurring piles of sodden debris and perhaps could also explain why this species seems well at home in compost piles in temperate regions.
Possibly it could also provide an explanation for incidents where Leucocoprinus cretaceus mushrooms are observed after flooding in human environments.
Observation 140409142 shows Leucocoprinus cretaceus mushrooms growing from the carpet in a house in Florida that was flooded after a hurricane. The owner of the property describes having 8 inches of level 3 contaminated water in the living room with black mold also being an issue. They did not return to the house until a month after the hurricane, which is when the photos were taken. It is of course possible that spores just washed in with the flood water, blew in after and settled on the damp carpet or that myceliated debris was brought in spreading the growth. Alternatively it seems possible that this growth could have been started by sclerotia washing in.
There are many observations of Leucocoprinus species growing from the walls and floors of properties on iNaturalist and it is not uncommon for ID requests to be posted on forums after people find the mushrooms growing from their buildings or furniture in tropical regions. Sometimes this appears to happen in temperate locations such as observation 16695204 made at some point during September of 2018 in Illinois, US. It is unknown if flooding was involved but it cannot be ruled out as a cause given the flooding which occurred in that area during May of that year as a result of subtropical storm Alberto[10] and during September as a result of thunderstorms.[11]
Observations 91736012, 69295193 and 90986172 also seem like cases where sclerotia could have found their way into these cracks to start growing, possibly via water inundation but where not enough information is provided to support this as spores could likewise be responsible. Observation 180854333 was made in Mexico at the end of August and shows a solitary mushroom growing beneath a stone slab used as planter with debris having washed into the corner from the rain. As such it is not clear if the fungus has been introduced with the rain via sclerotia or if it is just growing out from the planter. The prolific mycelial growth on the surface of the debris however may be indicative of the type of habitat it prefers.
This tendency to grow from cracks beneath skirting boards and doors is not unique to Leucocoprinus cretaceus with many observations of L. birnbaumii, L. cepistipes and other Leucocoprinus species growing in the same human environments. Likewise many species in the Psathyrellaceae family, Gymnopilus species and occasionally some Pluteus species amongst others may be found in this habitat. Such cracks and gaps may form ideal areas for humidity to build up to allow the formation of fruiting bodies and spores could readily find their way in there. As such it cannot be guaranteed that such growth is the result of sclerotia, merely it seems possible that they could help facilitate this behaviour.
If sclerotia serve as a means of asexual propagation then distribution via water would seem the most probable mechanism though further testing and observation would be necessary to determine whether this does happen in nature. Distribution of sclerotia via wind does not seem especially feasible or at least not as an explanation for why they have evolved in the manner they have. The sclerotia are small and light enough that they could potentially blow around like grains of sand in a desert or on a beach but in the dense canopy of a rainforest with the debris and plant cluttered undergrowth it seems doubtful that they would spread very far via this mechanism. Leucocoprinus cretaceus is also observed in North America, Africa and Australia but there do not appear to be any observations on iNaturalist of it growing in deserts, dunes or overtly sandy environments.
There are also many observations of L. cretaceus from Asia in locations with a similar tropical climate to South America and observations in the wild in especially warm regions in Europe also occur. Sequences suggest that this species has a global distribution and the consistency of the results would not appear to indicate any similar species. iNaturalist observation 135186560 from Florida by @lester34 shows a 99.70% match with observation 102740682 from Arizona by @jonaleef, a 99.54% match with observation 33746808 from Indiana by @dcchris and a 99.68% match with observation 91485340 from the Cayman islands by @jhaakonsson. 99-100% ITS matches to this Florida specimen are also found in collections from: Dominican republic, Phillipines, Malaysia, Laos, Thailand, China, India, Australia, Nigeria, Egypt. The lowest matches submitted under the name Leucocoprinus cretaceus are three sequences from Pakistan with two at 97.90% from the same study and 97.73% from another.
Another noteworthy submission is a sample submitted from Greece which is a 98.53% match to the Florida specimen but this is based on a query cover of only 81%. The sample was isolated from soil in an experiment on using the wastewater from olive mills as a fertiliser for pepper plants. The soil used in the experiment was loamy sand or sandy loam collected from olive groves which was first passed through a 3mm sieve. One finding of the experiment was that the Leucocoprinus cretaceus fungus appeared to be inhibited by the olive mill wastewater application.[12] It is also interesting to note that if sclerotia were present in this soil then a 3mm sieve would not be sufficient to remove them but as this sample is titled as 'uncultured' it is probable that the authors were not aware of sclerotia production in this species.
It is interesting to consider whether this apparent global distribution is due to anthropogenic factors or whether this spread occurred well before international aircraft and ship travel. As observations of L. cretaceus in plant pots are far scarcer than L. birnbaumii it would seem unlikely that distribution via potted plants and potting soil is the primary means in which it has spread. The sclerotia seem like a mechanism that is worth exploring to explain this distribution as air travel perhaps makes it inevitable that the species would spread globally via sclerotia. Tiny sclerotia produced abundantly on the surface of the soil seem ideal for getting stuck in the tread on shoes much as plant seeds do and so international tourism would make such a distribution method possible. However if sclerotia serve as an effective means of spreading in this manner then it can be expected that such distribution would have occurred prior to human travel.
It has been documented that spores can be distributed via birds on their feathers and as such it is already probable that migratory birds are responsible for spreading some fungi species globally. Although documentation of this appears to have been primarily with molds and some plant pathogen species[13] it would seem feasible that mushroom forming species could spread in the same manner. Whilst this could provide a plausible explanation for the spread of L. cretaceus globally it would also seem worth exploring whether the seed like appearance of the sclerotia results in any interactions with birds.
The peridioles of species in the Nidulariaceae family have been hypothesised to engage in seed mimicry resulting in birds eating them and distributing the spores further than could be achieved only by the peridioles being splashed out of the cup by rain water.[14] This has not been demonstrated in nature however but in captivity canaries were observed to consume the peridioles only if mixed in with other food and spores were capable of passing through the digestive system without damage. Digestion resulted in the peridioles breaking up and the spores being released.[15] Whilst the sclerotia of Leucocoprinus species are not spore bearing structures they are similar to the peridioles in their form, colour and hardness and so it seems plausible that a similar interaction with birds could occur. If mature, dried sclerotia were present in the top soil amongst scattered seeds it would seem possible for birds to incidentally ingest the sclerotia in the course of foraging.
Sclerotia are found in many plant pathogen species[5] and the size and appearance of these structures is often similar to seeds. Subsequently sclerotia may often be found on the ground in fields at the base of contaminated crops where fallen grain may also be present. Despite this it would appear that possible interactions between birds and sclerotia have not been well explored and as such it is not clear if such interactions even occur. A 2020 study notes that interactions between birds and fungi in general is not well documented with only 54 bird species documented as eating fungi. This study does suggest that birds serve as a distribution mechanism for fungi and also discusses the many brightly coloured, truffle-like fungi of New Zealand which are hypothesised to attract ground nesting birds with some species potentially mimicking fruit. Interactions between birds and subterranean mycorrhizal truffles in Africa and the Middle East have also been documented with animals, including birds hypothesised as distribution methods as a result of mycophagy.[16]
Compared to these species, Leucocoprinus cretaceus would not appear to have any specialisations that suggest an evolutionary forcing by bird distribution. Even if birds are not intentionally consuming sclerotia it seems possible that some sclerotia will incidentally be consumed by some birds in the process of foraging for insects, worms or slugs which are attracted to the mushrooms or living in the top soil amongst the mycelium and sclerotia. Depositing red wiggler worms from a wormery into a jar with spent substrate of Leucocoprinus cretaceus has demonstrated that sclerotia will stick to the worms' bodies in the same way as particles of soil or sand can. As the worms move, clumps of sclerotia stuck to them or solitary mature sclerotium free from the mycelial envelope are transported with them. This mechanism in itself could provide some distribution via sclerotia and could also be expected to occur with slugs and snails. It would seem unlikely however that such creatures by themselves would travel further than wind-borne spores could. It is not yet clear if the worms will eat the sclerotia or if they survive this process but sclerotia stuck to the exterior of the worms seems like a viable means in which birds may ingest them.
It is also not clear if or how well the sclerotia would survive digestion by birds and other animals although the structures have shown some resilience to chemical degradation such that it seems potentially possible. In this scenario however it could also be expected that spores from the mushrooms would be coating whatever nearby animal or plant material birds would be feeding on. The thick-walled spores of Leucocoprinus cretaceus may already be well suited to surviving digestion resulting in some amount of spread via birds though this may not be ideal. The addition of chicken manure to substrates appeared detrimental for the growth of L. cretaceus and resulted in very poor growth or no colonisation of the manure at all so this would not appear to be a preferred habitat.
The sclerotia are also likely to become stuck to the feet and fur of animals that walk over the colonised soil or forage amongst it. It would seem unlikely however that the sole or primary evolutionary purpose for the sclerotia is distribution via birds and other animals in this manner. If distribution via animals or interaction with them is a factor in the ecology of this species then the most likely candidates would appear to be ants or termites.
After the strongly aligned sequences listed above the next nearest matches are in the range of 91.69-92.21% and all are undescribed Leucocoprinus species isolated from the nests of fungi-farming Mycocepurus, Mycetosoritis and Myrmicocrypta species ants. Unlike the commonly known leafcutter ants in the higher attine genera of Atta and Acromyrmex these lower attine species do not farm Leucoagaricus gongylophorus and there is little information about the Leucocoprinus species they do farm. The studies for which these sequences were performed[17][18][19][20] do not mention sclerotia and so it is unclear if the Leucocoprinus species farmed by these ants produce them. A 2023 study on fungus growing ants in Brazil described two new species of Leucocoprinus with L. attinorum cultured from the nests of Mycocepurus goeldii and L. dunensis from the nests of Mycetophylax morschi. Unlike Leucoagaricus gongylophorus neither of these species have been documented as producing gongylidia. These new species are closely related to L. cretaceus and are now placed in the same clade.[21]
This photo and this one of Mycetosoritis hartmanni taken by Alex Wild does appear to show objects which look similar to sclerotia in amongst the mycelium. These may just be frass or debris though their similarity to the appearance of sclerotia in Leucocoprinus cretaceus is intriguing. Likewise the photo of Mycocepurus smithii carrying frass to dispose of looks oddly like the occasional conjoined sclerotia found in cultures of L. cretaceus.
Considering that the nearest known relatives of Leucocoprinus cretaceus appear to be species associated with ants and that the sclerotia are of a size, shape and texture that is similar to other objects the ants interact with and may likewise be appropriate for manipulation by ants it would seem prudent to explore possible interactions between them.
3.3 Association with ants or termites?
Reviewing observations of Leucocoprinus cretaceus from the wild in South and Central America turns up a few observations of ants eating the mushrooms with the pieces carried off by each ant being of a similar size to the sclerotia. Where the damage is extensive enough to litter the ground below with pieces of the mushroom it would not seem inconceivable for ants to collect sclerotia along with this debris. Interestingly observation 145529097 from Australia appears to show a mushroom with sclerotia on top of the cap, or at least structures that look very much like them. It is not clear how this came to happen but if heavy rainfall is sufficient to displace sclerotia from the soil with force enough to coat the cap of the mushroom or nearby leaves this could increase the opportunities for them to be collected when the mushrooms are harvested by ants.
As of yet it has not been explored whether ants are in any way interested in the sclerotia however it is worth noting that the smell upon opening a jar of cultivated Leucocoprinus cretaceus is very strong and that this smell appears to be from the myceliated substrate itself. In general this species has some very strange and powerful odours associated with it which raise the question of whether these are to attract animals like insects or mimic ant pheromones in some manner. Fresh mushrooms with caps that have either just opened or are about to open do not smell particularly of anything or are mildly pleasant, after opening they persist for a couple days before deteriorating at which point the smell becomes increasingly off putting with chemical or unpleasant floral notes. These smells change over time as the mushroom is left to dry naturally with it initially becoming more unpleasant day by day until the dried material develops a reasonably pleasant, savoury smell. The strongest smell however is in the myceliated substrate that is full of sclerotia and in the tiny, immature primordia. This is immediately noticeable upon opening a jar, even for a second inside a still air box whilst wearing a mask. It is neither especially pleasant or unpleasant but simply pungent and unusually like chemical aromas and this is likewise noticeable when sampling a small piece of the substrate which can be more pungent than a whole mushroom.
It therefore seems worth exploring whether this smell attracts the ants and affects their behaviour in any way. It seems unlikely that the ants would attempt to eat the mature sclerotia or collect them for food as the hardness is such that they may not be able to pierce the exterior layer. However if the sclerotia mimicked eggs or frass via their size, texture and smell this could result in the ants depositing the sclerotia in an environment optimal for growth such as a nest or waste pile.
 |
|---|
| Observation 138624470 - L. cretaceus on possible ant debris Credit: @annie_tremblay |
Whilst this review was carried out in order to look for signs of possible association with ants it found few observations that showed any sign of interaction. However it did turn up a significant number of observations from termite mounds, pieces of arboreal termite nest that have fallen to the ground and wood with signs of termite damage or termite mud tubes. Many observations in which the mushrooms are growing from holes in the wood or from wood with holes in it might be explained by beetles with the spores either being caried in by the insects or drifting into the holes. Observations in which termite mud are present however are interesting as L. cretaceus would likely grow faster on this substrate than on the wood itself and then be able to spread into the wood from the mud. Observations in which some signs of termite or ant association may be present have been collected in a project to compare with a broad approach applied so as not to exclude observations where it is unclear but possible. Observations on termite mounds have also been found in Africa and Australia and are likewise included in the project.
Mushrooms growing on termite mounds is of course far from unique, the substrate is evidently highly nutritious and many species can be observed growing on old mounds. Termitomyces and Podaxis are known to be associated with termites but all manner of saprotrophs can be observed growing on termite mounds when browsing fungi observations in regions with a lot of termites. Additionally standing or fallen wood that is peppered with termite holes is going to be naturally prone to colonisation by fungi either from spores spread via the wind or brought in by insects. A review of observations for a wood loving genus like Gymnopilus will likewise turn up many observations growing on termite damaged wood.
It could therefore be easy to ignore any possible association between termites and Leucocoprinus cretaceus and just dismiss it as another opportunistic saprotroph whose spores happened to end up there. The sclerotia however may provide an alternative explanation which should be seriously considered as there is a species of fungus associated with termites which produces sclerotia of a similar size and shape to those of L. cretaceus in order to engage in egg-mimicry.
 |
|---|
| Observation 149068086 - Athelia termitophila 'termite balls' fungus Credit: @kim_fleming |
Athelia termitophila is a fungus that produces sclerotia, commonly known as 'termite balls', which have been extensively documented from the nests of Reticulitermes species termites where they mimic eggs resulting in the termites tending the sclerotia. They were initially described by Kenji Matsuura as the sclerotia of a Fibularhizoctonia species considered to be an anamorph of an Athelia species.[22] The sclerotia are globose, starting white before maturing to pale brown, orangy-brown or brown with a size of 240 - 410 μm.[23] Rarely oval shaped sclerotia are formed due to the fusion of two sclerotium,[22] a trait that is also seen rarely in Leucocoprinus cretaceus sclerotia.
The termite ball sclerotia use a chemical signature to mimic the eggs and also mimic their size and shape with sclerotia that are of a similar diameter to the eggs being tended by the workers whilst smaller ones are not. The globose shape of the sclerotia does not match the longer, oval shape of the eggs and so they appear very different to the human eye but as workers carry eggs via the shorter side the sclerotia apparently do not need to match the shape in order to trick the termites.[24] Likewise the colour would not appear to be of any importance since termite workers are blind[22] but the texture may be a factor as both the sclerotia and eggs are similarly smooth. There may therefore be a selective pressure for the fungus to produce sclerotia that are similar in size, shape, smoothness and chemical signature to the eggs but no pressure for colour.
This mimicry prompts the termite workers to pile up the sclerotia as if they were eggs and tend to them by keeping them moist with saliva. The behaviour appears to inhibit germination of the sclerotia with fewer germinating in the presence of the termites than those left untended.[24] Whilst experiments without nest material initially suggested that the sclerotia improved the survival rate of the eggs, possibly due to them inhibiting fungi or bacteria[24] it was later concluded that this seemed unlikely to occur in the natural environment with nest material already providing some antibiotic effect.[22] Rather than having a mutually beneficial symbiosis with the termites the fungus appears to be parasitic. Whilst the fungus only rarely kills and colonises the eggs, the number of sclerotia is sometimes greater than the number of eggs in a nest subsequently resulting in time and energy being wasted by the colony for no reward. The termites do not consume the sclerotia or obtain any nutritional benefit from the presence of the fungus.[22]
Basidiomes of the fungus appear to favour cooler temperatures with observations of them in nature taking place between 3.9 - 17.5°C whereas the sclerotia are produced, in culture, at 15-30°C. The sclerotia therefore may serve as a survival mechanism for warmer temperatures when fruiting bodies cannot be formed.[23] During colder months most termites burrow underground with egg production ceasing resulting in the sclerotia being ungroomed by the workers, thus removing the inhibition to germination from the saliva. The fungus shows a high tolerance to cold and may then be able to colonise the decaying wood in the absence of the termites.[25]
Temite balls have been found in the nests of Coptotermes formosanus, Reticulitermes amamianus, R. kanmonensis, R. miyatakei and R. speratus in Japan, R. labralis in China and R. flavipes and R. virginicus in the United States.[23]
iNaturalist does have a couple observations of R. flavipes in South America but otherwise these species and others in the Reticulitermes genus are not found there and are not native to this location. With Athelia termitophila appearing better adapted to cooler temperatures it would seem ill suited to this environment. South and Central America do have many species of termites distributed across many genera with a wide variety of nesting behaviours however. Given the number of observations of Leucocoprinus species on nests and the likelihood of more Leucocoprinus species producing sclerotia which are as yet undocumented it would seem prudent to consider whether a similar relationship may exist which fills the same ecological niche. Alternatively the presence of L. cretaceus mushrooms fruiting from nests may be explained by the properties of the termite mud material.
Observations in which Leucocoprinus cretaceus is fruiting from termite mud tubes or the remnants of them on trees appear to be quite common. In culture L. cretaceus performs well on potting soil and benefits from the addition of plant material mixed into the soil whilst growth directly on wood is slower. Termite mud tubes are made of soil and can contain fecal matter and organic material mixed with termite saliva whilst Nasute termites build tubes out of 'carton' material.[26] The carton material that arboreal termite nests are built from has a consistency like papier-mâché with an interior made of chewed wood and an outer wall of hard soil material. Termite fecal material and saliva are used like cement to bind material together.[27] This may form optimal conditions for L. cretaceus to grow with the termite holes in the wood then providing an easy ingress for the fungus to begin to colonise the wood.
A study on termite nests in Indonesia that sampled three sites found a carbon content in the nests of 2.82, 3.05 and 3.46% showing an increase compared to the 2.08, 2.21 and 3% in the surrounding soil. Whereas the nitrogen content was 0.23, 0.24 and 0.25% in the nests compared to 0.27, 0.15 and 0.20% in the soil. The additional carbon was derived from the feces and saliva of the termites as well as organic matter which was higher in the nest material than in the surrounding soil. The nest material also contained a higher moisture content than the surrounding soil owing to the higher quantities of organic matter and cellulose mixed with the clay from soil.[28]
It's probable that spores from L. cretaceus would germinate and grow well in this environment and so colonisation of this termite mud may simply be explained by this mechanism. Whilst L. cretaceus does have many observations growing on nests it is not unique in this behaviour and other species such as Leucocoprinus parvipileus and L. tephrolepis were documented growing on old nests[29] and other species are observed in this environment with casual browsing on iNaturalist.
However as the sclerotia are of a size that could be carried by termites it would seem conceivable that they could be picked up and placed in the mud during nest or tube building behaviour. This would likely be difficult to observe in nature as the mature sclerotia have a colour and texture that would make them difficult to find amongst the termite mud. Additionally if the material has been colonised by spores of L. cretaceus then some sclerotia will almost certainly grow before any mushrooms do. When old termite mud tubes and carton material break apart and fall to the ground sclerotia and myceliated substrate could be distributed into the soil and leaf litter below. Whereas sclerotia produced in hollows in wood would be less likely to be distributed. This may provide an explanation for why L. cretaceus appears to produce few to no sclerotia directly on wood but many on soil material or more nutritious substrates whereas the fruiting bodies are produced adequately on wood, soil and high nutrient substrates alike.
As sclerotia will grow in the light and appear to favour the surface of substrates it could be expected that sclerotia would be produced on the exterior surface of termite mud tubes or carton material and then fall to the ground below as they mature and become free of the mycelial envelope. In this manner some limited wind distribution might be expected before they hit the ground, especially with the smallest sclerotia. Since sclerotia are produced continually as the mycelium spreads resulting in sclerotia of all levels of maturity being present simultaneously the colonisation of elevated termite nests may result in a steady supply of sclerotia raining down on the surrounding area.
In order to assess whether any such relationship with ants or termites exists it would be necessary to see if/how workers interact with sclerotia during different stages of maturity and whether the strong smell of the myceliated substrate alters their behaviour in any manner.
4. Conclusion
The sclerotia of Leucocoprinus cretaceus may confer a number of advantages rather than performing only one specialised function. As the sclerotia naturally dry out during maturation it would seem probable that they are able to survive periods of drought or cold and remain viable for extended periods of time in the soil. Further experimentation should reveal how long they can persist in this state and what extremes of temperature they are able to survive.
It is unclear how abundantly sclerotia are produced in nature compared to in culture or to what extent distribution via water may be a factor. However the basic physical properties of the sclerotia would suggest that some sclerotia will invariably be relocated via water flow. Further experimentation with simulated flooding and inoculation of non-sterile material via sclerotia will be necessary to determine how viable this distribution mechanism is.
The experiments detailed here with mold were unplanned and the result of accidental contamination. Some controls were present by virtue of the other jars that remained uncontaminated but it would be preferable to assess the ability of sclerotia to survive contaminants via more detailed experimentation as well as the inoculation of substrates with multiple competing species. It would appear that the sclerotia confer some ability to survive mold which could further aid survival during dormancy in soil or improve their ability to survive competition on fresh substrates after flooding.
Sclerotia production appears to be increased in higher nutrient substrates though sclerotia size, whilst quite variable, appears to be in a consistent range regardless of substrate. In nature sclerotia could be expected to be produced more abundantly in soil, termite mud or on nutritious plant material than on wood. The impact of temperature and light on sclerotia production still needs to be explored and doing so in conjunction with another species which forms similar sclerotia such as Leucocoprinus cepistipes may be prudent so as to compare the ecology of these species.
Relationships with ants or termites are entirely speculative at this time. Ideally the experimental methodology outlined in the studies on Athelia termitophila in which termite workers are exposed to sclerotia and similarly sized synthetic objects could be repeated for Leucocoprinus sclerotia. Accumulating more observations in the wild would also be preferable and it would seem probable that sclerotia will be found if colonised termite mud material is dried, crumbled and sieved.
As the sclerotia are produced so readily and in such vast numbers on a variety of substrates it may be worth exploring possible applications for them such as carbon sequestration. It would first be necessary to determine the carbon content of the sclerotia in order to assess viability for this purpose. If they do contain a significant enough carbon content it would seem quite viable to lock away carbon from decomposing organic material in the readily cultured sclerotia. The irregular shape, size and texture of the sclerotia could make for a sand like aggregate that would be easily bonded by resin to form construction material.
References
as a predictor of susceptibility to Coniothyrium minitans?". Biological and integrated control of plant pathogens, Vol. 133, pp. 181-186.