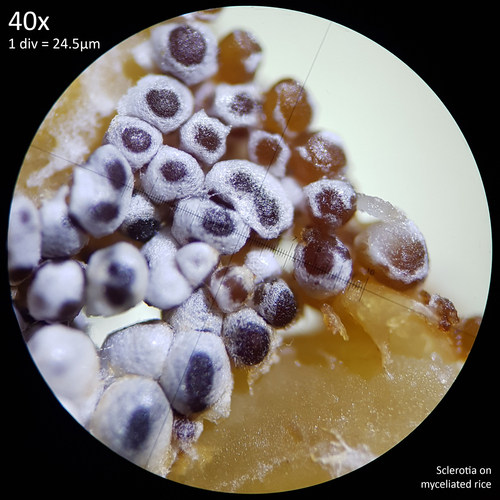
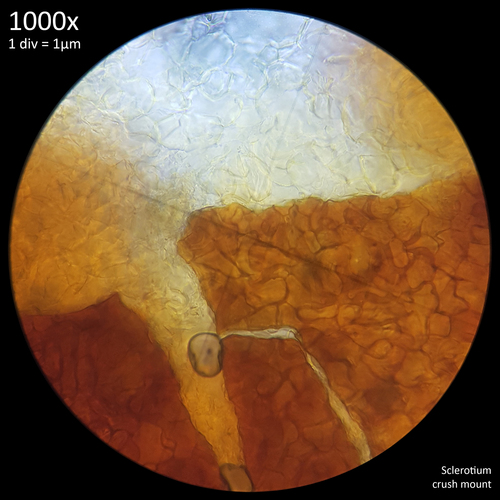
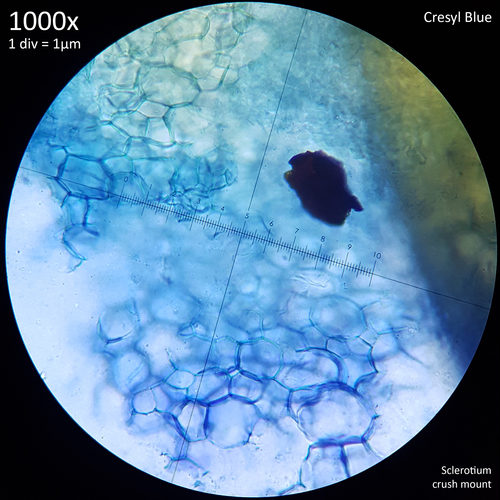
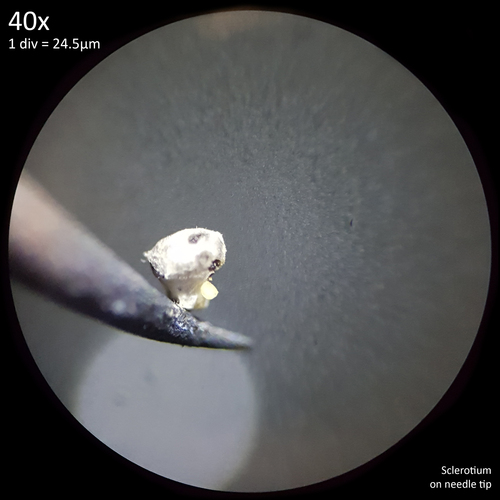
Leucocoprinus cretaceus - An edible mushroom?
Abstract
Leucocoprinus species are generally not counted amongst the edible mushrooms that can be safely foraged or are worthwhile to cultivate. Their superficial similarity to toxic Lepiota species and the known or presumed toxicity of L. birnbaumii and L. brebissonii may have resulted in suspicion of other members of this genus. However reviewing the literature on Leucocoprinus cretaceus will turn up many mentions of its edibility, lack of toxicity and even its pleasant flavour. A review of this literature is presented here with several erroneous entries in the taxonomic history highlighted and a number of synonyms suggested. The culmination of this research was the determination that L. cretaceus is indeed safe to consume and to this end it was cultured, cooked and consumed numerous times without ill effects. It is not only a very pleasant tasting mushroom with a satisfying, meaty consistency but it is also easily grown on a variety of substrates including cheap, commercially available potting soil and coir. Given that this species prefers warm climates it may represent a viable edible species to culture in regions where conventional fungiculture is limited by high temperatures. Additionally with Summer temperatures increasing globally and many conventional agricultural practices suffering as a result it may be worth considering for seasonal cultivation in temperate regions, for cultivation in greenhouses alongside edible plants or small scale cultivation at home as growth at room temperature can be adequate. In order to undertake cultivation it is necessary to understand the abundant sclerotia produced by this species and as of yet their edibility is not known and careful effort has been made to avoid consuming them.
1. Introduction
The taxonomic history of Leucocoprinus cretaceus is filled with mentions of edibility and descriptions of it smelling and tasting very pleasant. This species however has been surrounded in confusion ever since it was first described and has regularly been conflated with L. cepistipes, L. birnbaumii and others. As such these accounts are unreliable especially as they are in contrast with more recent and reliable descriptions that note an unpleasant smell and taste. In 'Flora Agaricina Neerlandica' Vellinga notes that descriptions of the smell are variable with it having been recorded as weak, Lepiotoid, similar to that of Inocybe bongardii or strong, unpleasant and nauseating even in young specimens. A herbaceous smell similar to geraniums (Pelargonium) is noted when crushed and this comparison has also been made by other authors with some Inocybe species. The taste is likewise described as strong, unpleasant and astringent though not bitter. A spore size of 8.0-12 x 5.5-7.5 μm is given or 8.7-10.5 x 5.9-6.8 μm on average.[1]
One explanation for the variation in the smell noted by different authors may be due to the confusion over identifying this species since even some modern papers and books still conflate it with L. cepistipes. It may also be worth considering the variation in how different people perceive smells and tastes since there are many species with descriptive smells noted by some authors whilst others note nothing discernible or simply say it is indistinct. Even the strong, pleasant aniseed smell of Agaricus arvensis or unpleasant phenol, inky smell of the cut flesh of Agaricus xanthodermus are not noted by some observers rendering these methods of identification unreliable.
However this experiment in culturing L. cretaceus has revealed that the mushroom simply does have a very variable smell depending on the maturity of the specimens and which part is tested. It does indeed range from insignificant, pleasant, floral and simply unpleasant or even nauseating depending on the age and condition of the specimen. The result is a mushroom that varies substantially between smelling appetising and highly off putting.
Whether or not Leucocoprinus cretaceus is edible seemed worth exploring out of more than simple curiosity - many cultivated mushrooms are species which would fruit naturally in Autumn, preferring cooler environments which may not be easily created in some locations. Whereas L. cretaceus is noted to thrive in hot and humid environments with it often appearing in greenhouses and indoor plant pots during the summer. With climate change rendering the Summer months increasingly hot and hostile to conventional agricultural practices it seems important to try and assess which tropical species can be cultivated for consumption. Leucocoprinus species already do well in the environments we have created, routinely appear in plant pots and are quite versatile saprotrophs with observations on soil, leaf litter, wood and in compost. As such they seem ideal for cultivation.
In order to explore the edibility of L. cretaceus it is necessary to first address some possible synonyms which suggest edibility before going into the further taxonomy and historic literature.
2. Possible synonyms
Genbank sequences for Leucocoprinus cretaceus are very consistent with many sequences with a 99-100% match and no outliers. Whereas what is seen with Leucocoprinus birnbaumii is a much wider range with many at only 90-95% suggesting other species submitted under the same name. Other Leucocoprinus species also have more of a range and none seem to have so many consistent samples as L. cretaceus.
It therefore seems likely that the three species listed here, all of which are regarded as current species in both Species Fungorum and Mycobank, should be considered synonyms of L. cretaceus rather than being something that is merely similar. The species described by Beeli, Heinemann and Bouriquet make no mention of any of the synonyms of L. cretaceus so do not appear to have considered this species when describing their own. The species described by Smith and Weber is compared to a number of species that are considered synonyms though there appears to be no significant distinction between their species and L. cretaceus. Subsequently the information they present regarding the taste, smell and potential edibility seem like they may be applicable to L. cretaceus.
Leucocoprinus elaeidis
Lepiota elaeidis (or elaidis) was described in 1927 by Maurice Beeli from DR Congo where they were found growing in groups at the base of palms. The description and illustration of an all white mushroom suggests that it may be synonymous with Leucocoprinus cretaceus. The spore size given is a match and the cap and stem are described as white and covered in flakes or scales with a yellow discolouration noted on the stem when touched or damaged. It also notes yellowing of the gills and sometimes a yellow or brown tinge to the cap. This correlates with many observations of L. cretaceus that show yellow on the stem if the white covering is removed or some yellow-brown discolouration towards the centre of the cap. This can routinely be observed during cultivation of L. cretaceus with the yellow colouration developing in old specimens but being absent in younger ones. Beeli says the locals called the mushroom elela and so it was evidently known to them but no information on edibility is mentioned. The smell and taste are described as pleasant however. Beeli gives a spore size of 9-10 (12) x 6-8 μm.[2] The species was illustrated by M Goossens-Fontana in Paul Heinemann's Flore Iconographique des Champignons du Congo[3] and Heinemann would later go on to reclassify this species as Leucocoprinus elaeidis, providing a spore size of 8.5-11 x 5.3-7.4 μm.
Leucocoprinus nanianae
Leucocoprinus nanianae was described from Madagascar by Gilbert Bouriquet in 1946 but this species seems poorly known as the text it is found in appears to be quite rare. However it likewise sounds like a synonym of Leucocoprinus cretaceus with a similar description of a white and powdery cap and stem and a spore size that again is a match. The cap is noted as having some very light brown fibrils but the illustration appears to show yellowing at the centre of the cap and possibly elsewhere around the gills and stem, though this is not noted in the text. The taste and smell are described as pleasant and some of the natives said the mushroom was edible so Bouriquet fed 400 grams of the cooked mushroom to a dog, which apparently did not suffer any ill effects. A spore size of 8.5-12.25 x 5.5-8.5 μm is given.[4] In 'Nomenclatural Overview of Lepiotaceous Fungi (Agaricaceae)' Vellinga notes that it looks like L. cretaceus.[5]
Leucocoprinus breviramus
Leucocoprinus breviramus was described by Helen Vandervort Smith and Nancy Smith Weber in 1982 from specimens collected in Brazoria county, Texas. The species is described very similarly to Leucocoprinus cretaceus noting the white colouration, floccose scales and a slightly yellowish brown tinge in the centre of the cap. Yellow staining is noted when the stem is handled with a floccose coating below the annulus and a smooth surface above. The specimens studied were collected by Ervin Hillhouse in 1971 and he regularly ate them describing the raw taste as mild and the cooked taste as very good and like that of young button mushrooms. Even with large servings no symptoms were noted. A spore size of 7.5-9.0 (10) x 5.5-6.5 μm is given.[6]
The reasons for Smith and Weber describing this as a new species rather than recognising it as L. cretaceus appear to once again come down to the confusion in the early taxonomy of Leucocoprinus species and the erroneous illustration provided by Bulliard. Smith and Weber note that their species does not resemble Bulliard's illustration of Agaricus cretaceus but assume that the illustration is the type for the species. They say that their species is closely related to Lepiota cretata Locquin giving a citation of Haller, 1950.
Haller refers to Lepiota cretata Locquin 1949 citing letters sent to him by Marcel Locquin.[7] However Locquin had already described the species as Leucocoprinus cretaceus in 1945 so the reason for the difference in name is unclear.[8] Haller says it is similar to Bulliard's illustration but fluffier and whiter noting that Bulliard's somewhat grey illustration does not correspond to his description but that good illustrations are found in the work of Mattirolo and Cooke. He notes L. cepaestipes as being a collective species with the white, brown and yellow forms. The photo that is included is easily recognisable as L. cretaceus even in black and white.[7]
Smith and Weber also cite the 'excellent description and discussion on L. cretata' that is presented by Marcel Josserand. Much of the history and taxonomic confusion is discussed here but Josserand remarks that the species is so distinctive that it unclear how it ever got confused. Josserand rejects Bulliard's illustration as representing the species and agrees with Locquin and Haller on the identity of Lepiota cretata. A discrepancy is noted in the description of the taste with Haller noting it as bitter but Josserand describing it as sour but not bitter, though noting it is not a serious discrepancy.[9]
However Smith and Weber reject both Bulliard's illustration and Lepiota cretata for reasons that are not entirely clear. They do however note that Leucocoprinus cretatus Locq. ex Lanzoni appears to be a European species which differs by virtue of being more robust, fruiting in large clusters, having firm, hard flesh when young and a bitter taste. They also note a larger spore size. This may be worth exploring further however I have yet to manage to locate this text and these differences do not seem significant. In culture it can be observed that sometimes singular fruiting bodies of L. cretaceus will mature and other times vast clusters together. The flesh of the stipe is very firm when fresh but becomes soft within a few days and when conditions are colder the mushrooms appear more prone to growing short and thick or aborting entirely rather than displaying the more slender appearance. Temperature and humidity may also be factors in whether a single large mushroom grows or many small caespitose fruiting bodies form. An appreciable difference in spore size can be noted if comparing a spore print to a gill section as it appears that many larger spores in some Leucocoprinus species may remain attached to the gills rather than falling when spore printing them.
Smith and Weber do not compare their species to Leucocoprinus cretaceus (Bull.) Locq. (1945) and the text in which it was described is not listed in the references so it is unclear if they were aware of this name amidst all the other competing and confusing names. In the nomenclatural overview, Vellinga notes L. breviramus is 'probably synonym of Leucocoprinus cretaceus'[5] Additionally in Nancy Smith Weber and Alexander H. Smith's 1985 book 'A Field Guide to Southern Mushrooms' a photograph of Leucocoprinus breviramus is provided and clearly shows L. cretaceus.[10]
It therefore seems probable that the species which Ervin Hillhouse consumed, often in large servings, was Leucocoprinus cretaceus. This seems to provide good supporting evidence for the species being edible but similar accounts are found in the taxonomic history of this species.
3. Taxonomic history
Like many of the most common Leucocoprinus species the taxonomic history of L. cretaceus is confusing and full of misidentifications and erroneous information and so requires significant time to unpack and evaluate. The species presented here are ones that are currently listed as synonyms in Species Fungorum and/or Mycobank and which include mentions of edibility. Whilst it was the mention of edibility in some of these species which provided the impetus to explore this subject some of these synonyms are clearly erroneous when the texts are further investigated.
Agaricus cretaceus
In 1788 Jean Baptiste François Pierre Bulliard described Agaricus cretaceus from greenhouses in France and said the mushrooms were very pleasant to smell and taste. However the illustration provided more closely resembles Leucocoprinus cepistipes so it is not clear which mushroom he was tasting.[11] This illustration would go on to create much confusion with Agaricus cepaestipes often being used interchangeably with this species, however as that species itself was often illustrated as yellow like L. birnbaumii the confusion was only exacerbated and all three species were often considered to be the same.
In 'Flora Agaricina Neerlandica', Vellinga notes that Bulliard's description clearly notes a cottony, chalk white mushroom which does not confer with the illustration resulting in some authors arguing that this illustration represents Leucoagaricus leucothites. Subsequently some authors have used Leucoagaricus cretaceus as a name for Leucoagaricus leucothites.[1]
It is possible that this confusion may have resulted in synonyms being listed for Leucocoprinus cretaceus that would make more sense as synonyms of Leucoagaricus leucothites (now known as Leucocoprinus leucothites.)
Pluteus cretaceus
In 1836 Elias Magnus Fries described Pluteus cretaceus and said they were edible and tasted better than other mushrooms. A common name of Krithvita champignonen was given which translates as 'chalk white mushroom'. Species Fungorum and Mycobank list this as a synonym of Leucocoprinus cretaceus however the description that Fries gives makes this doubtful.
The description itself provides little more detail beyond it being a completely white, edible mushroom with wide gills which retain their white gill colour until the mushroom starts to degrade. The stem is described as hollow, with a ring and no volva. It was found growing around Lund, Sweden in late Autumn.[12]
It seems unlikely that the tropical L. cretaceus would find late Autumn in Sweden to be a satisfactory environment in which to fruit but Fries does not specify any further details on the habitat, like if they were in greenhouses or compost. The more important information may be the gills retaining their colour until they start to degrade. This species is listed alongside Pluteus bombycinus and Pluteus campestris and the section on the Plutei is introduced by saying these species have reddish or brown spores but do not deliquesce into black liquid like the 'completely useless' Coprinoids. It would appear then that Fries was grouping Volvariella bombycina and Agaricus campestris (for which he notes several similar species exist that are not important to separate) together with Pluteus cretaceus because of the whitish caps and pinkish gills. When he notes that the gills retain their white colour until they start to degrade he may be describing a species with white gills that discolour pinkish with age.[12]
This suggests that Fries was actually describing Leucocoprinus leucothites, an all white mushroom which develops a pink colour to the gills with age. This species is often regarded as being edible with some sources cautioning a gastrointestinal reaction in some people or not recommending them due to the potential for novice foragers to confuse them with dangerous Amanita species. It does not explain the reddish brown spores noted but perhaps Fries simply did not spore print it and was only grouping it together with these species based on the similar appearance and gill colour. In any case it seems a far more likely candidate to find in Sweden in Autumn than L. cretaceus. Subsequently Fries' description of this as an edible and tasty mushroom must be disregarded for this research.
Psalliota cretacea & Pratella cretacea
In 1871 Paul Kummer described Psalliota cretacea as growing in fields, meadows and gardens in Germany in Autumn. He stated that the mushroom was tasty and suggested the common name Kreideweißer champignon which translates as 'chalk white mushroom'. He does not state whether he is reclassifying Fries' Pluteus cretaceus though his description is also more likely to be Leucocoprinus leucothites as many of the species he is describing as Psalliota are now considered Agaricus species. His key to the Psalliota genus notes gills that start pale or grey before blackening, turning faint olive green, reddish brown, purplish or brown.[13]
In 1878 Claude Casimir Gillet reclassified Fries' Psalliota cretacea as Pratella cretacea agreeing that the flavour was pleasant and said they were an edible mushroom. Gillet's description for the Pratella genus says they have brown or purple-black spores and most of the species he describes and illustrates within this genus have since been reclassified as Agaricus.[14]
Therefore Kummer and Gillet's description of the species as edible should also be disregarded here. It isn't clear if these species ended up listed as synonyms due to a mistaken reclassification or because of the former classification of Leucoagaricus leucothites as Leucoagaricus cretaceus. Regardless they do not appear to be descriptions of Leucocoprinus cretaceus.
4. Further descriptions
1835 Carlo Vittadini describes Agaricus (Lepiota) cretaceus with a description that seems accurate and correct for this species. It is described as growing in groups on rich substrates comprised of decomposing animal and vegetable matter, so presumably was observed on compost. Vittadini does not specify that they were found in a greenhouse however with the temperatures in Italy it wouldn't be surprising to find them growing outside. He says the mushrooms are edible and similar in taste and smell to Pelliccione, the common name given for Agaricus procerus, now known as Macrolepiota procera.[15]
E affatto innocente, e raccolto giovinetto può essere, al pari del Pelliccione, per gli usi della tavola destinato.
'He is completely innocent and collected as a young man, like Pelliccione, he can be destined for the uses of the table.' (Translated from Italian via Google).
However a strange footnote is included:
(t) Donnèe aux animaux, à la dose d'un seul , elle excite deux heurss après un vomissement considérable j l'animai se piami, ne veul rien prendre , tombe dans l'assoupissement et meurt. Paul. 2, pag. 360.
This is taken from Jean-Jacques Paulet's 1793 text Traité des champignons.[16] (Translated from French via Google).
XXIV. The Palette with darts or three-carts (pi. CLXIII, fig. 3). This species, which I do not find described, is a mushroom which rises to the height of five or six inches, white, but with [gills] which take on a green eye. The surface of the [cap] is white & covered with triangular, equal, pyramidal points; these points of a dirty white, adhere strongly by their base to your skin which covers the [cap]. This [cap] is regularly circular. The [gills] are usually covered with a dust similar to fine flour, and with a fine veil which ends up hanging only on the stem, and acts as a collar. The stem is white, cylindrical, of an equal diameter, full of a soft substance furnished by a bulb which pivots a little in the earth, and is exhausted, it becomes hollow like the stem.
This plant, which one finds in autumn in the park of Saint Maur, is of a soft substance, & has a very pleasant odor of ordinary mushroom; however, it is very evil. Given to animals, in a single dose, it excites considerable vomiting two hours after; the animal complains, does not want to take anything, falls into drowsiness & dies.
Paulet's text is filled with mentions of him feeding various mushrooms to animals to see if they would die... but it is not clear which animals were the unfortunate subjects of his questionable tests. Searching the ~500 page text for 'animaux' turns up 197 results, 'chien' turns up 33 and a quick look at some of these shows he was indeed deliberately feeding mushrooms to dogs to see if they would die. Other searches for common test animals like rats, mice and rabbits don't turn up results and there were only a couple mentions for cats. So it could be that the animal which died from eating this mushroom was a dog.
The green discolouration mentioned in the gills is interesting. This immediately suggests Chlorophyllum molybdites, which the size and scaly cap would support however this is generally regarded as a North American species. A study in 2022 noted that Chlorophyllum molybdites was widespread on the coast of Sicily in Italy[17] and an online post from the University of Veterinary Medicine Budapest cites identifications of the species in Scotland, Australia and Cyprus with the first poisoning cases being in Sicily and Spain in 2014.[18] though citations are not given to explore further.
It doesn't seem impossible that the species could have found its way to Europe in the late 1700s and that may explain the author being unable to find a description of it however the base isn't especially bulbous as noted in this description and combined with the pyramidal scales it starts to sound more like an Amanita species.
Whilst C. molybdites is regarded as highly toxic with serious gastrointestinal distress, it generally is not considered as fatal with no such incidents recorded in adults. However there are a few isolated cases of small children dying after consuming these mushrooms and many sources mention that they may be deadly to dogs and horses. This information is circulated on several sites but the original source of it is unclear so it cannot be evaluated for veracity.
Another possibility is Leucocoprinus cepistipes, which is sometimes observed to develop greenish gills with age or upon drying and Leucocoprinus cepistipes var. rorulentus specifically does note this trait. The bulbous base would match, the height of the stem given is within the range described and this species was documented from Europe. There is no information on toxicity with this variant but it seems doubtful that it would be fatal if consumed however.
Paulet gives no scientific name for the mushroom he is describing and instead provides only a common name of La Palette à dards ou à trois-carts and a reference for an illustration on plate CLXIII, fig. 3. The other mushroom described on this page is given the common name La petite Rape and is illustrated in fig. 1 & 2.[16] These plates do not appear in this text however, at least not in the scanned versions available online. Other species in this text correlate with the illustrations in Iconographie des champignons de Paulet[19] but the common name given does not for this mushroom. Plate CLXIII figures 1 & 2 have the common name Oronge à pointes de rape and the scientific name Hypophyllum radula. Figure 3 has the common name Oronge à pointes de trois quart and the scientific name Hypophyllum tricuspidatum. The citation for each points back to page 359 in Traité des champignons so does confer with the previous text.
To further confuse the matter page 89 of Iconographie des champignons de Paulet gives a description for these illustrations and gives the name Agaricus echinocephalus[20] (now known as Amanita echinocephala). This species is known from Europe and descriptions do note a slight greenish tinge to the gills so this would seem to be a good contender for the species described by Paulet.
Neither Hypophyllum radula or H. tricuspidatum are listed in Species Fungorum with any synonyms and Mycobank lacks a full citation for either but lists H. radula as having the current name Agaricus pauletii (no page citation) with the later classified Lepiota pauletii listed as a synonym. The citation for Lepiota pauletii however does not seem to point to anything by this name but does include an illustration of Lepiota echinocephala.
Further time has not been spent exploring this because it seems that regardless of which species poisoned the dog (or rather which species Paulet poisoned the dog with), it does not match the description of Leucocoprinus cretaceus and so Paulet's description of toxicity can be disregarded. Vittadini's information however on edibility is useful nonetheless as his own description matches that of L. cretaceus.
In 1918 Oreste Mattirolo described Lepiota cretacea as a reclassification of Bulliard's Agaricus cretaceus. He did not provide a description however and instead referred to that provided by Vittadini which he compared to 'a photograph made with words'. Mattirolo says the similarity to Pelliccione, Lepiota procera as described by Vittadini is remarkable. Mattirolo says he collected them for food but it is not clear which species he is referring to and whether the 'remarkable' similarity he is noting is in reference to the taste or appearance.
Translated from Italian via Google:
In order to have an idea of the type of our mushroom, it seems to me very opportune to mention the observation made by Vittadini, that the Cretaceous Lepiota resembles the common Pelliccione, that is, the Lepiota procera Quel. Having collected in the soil deriving from the decomposition of the leaves in the Royal Botanical Garden of Turin, numerous specimens, which I also used as food, I was able to convince myself that, especially in the more developed individuals, this similarity is remarkable.
It sounds like Mattirolo was eating Lepiota cretacea but it is unclear. The only description he provides personally are measurements of the spores at 8-10 x 4-6 μm which is within the correct sort of range for Leucocoprinus cretaceus.[21]
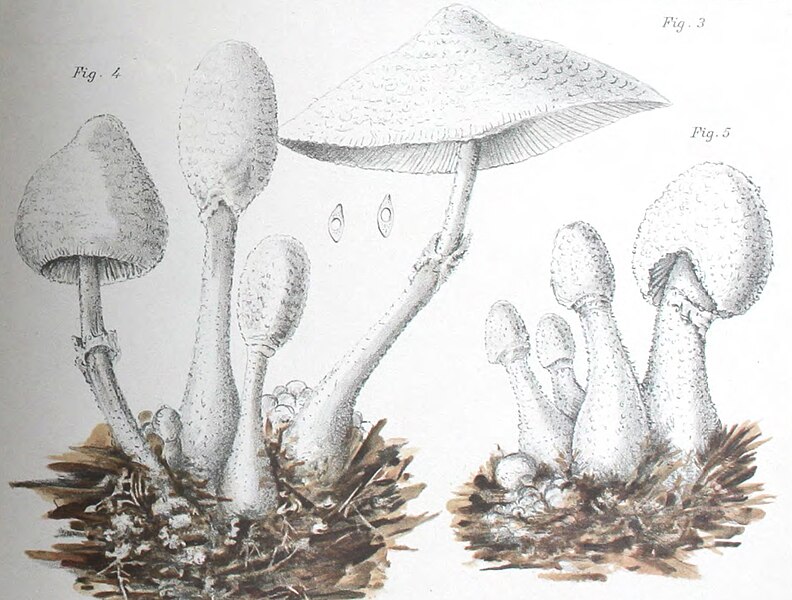 |
 |
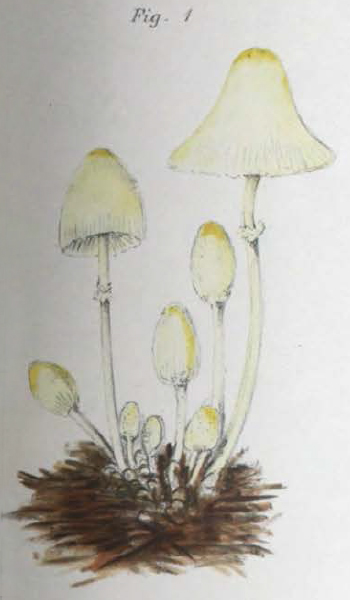
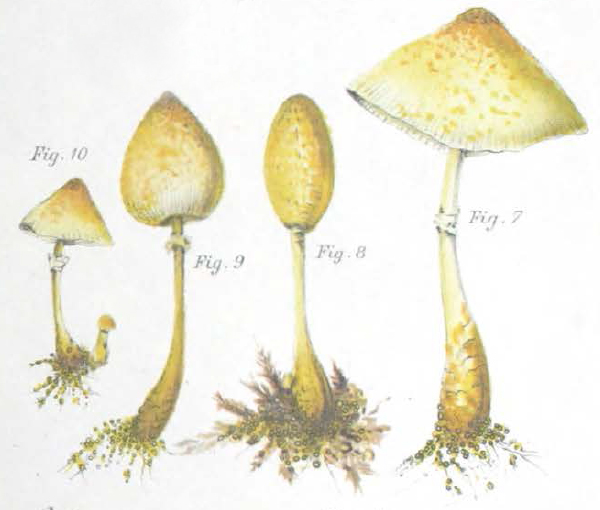
| Fig. 1 - Mattirolo's illustration of Lepiota cretacea (1918) | Fig. 2 - Hard's photograph of Lepiota cepaestipes (1908) |
Mattirolo's illustration (fig. 1) is also a match for Leucocoprinus cretaceus and could not really represent anything similar like L. cepistipes as has been confused by other authors. Mattirolo also provided a list of illustrations which he said best compared with Lepiota cretacea. Fortunately these are all long since out of copyright and so they can be included here to easily compare side by side.
Figure 2 - Miron Elisha Hard's photograph of Lepiota cepaestipes [22] in his 1908 text 'The mushroom, edible and otherwise' is described by Mattirolo as 'very significant'. With the scales visible on the stipe it does seem to resemble L. cretaceus more than L. cepistipes. Hard lists it as an edible species however he also describes there being yellow, yellowish and white forms so it appears that he was unclear on the identity of these species.
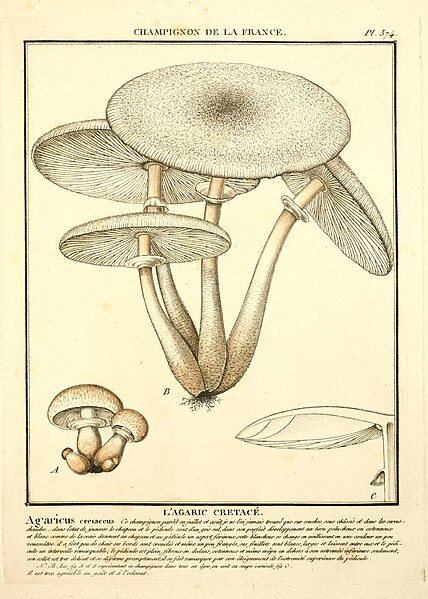 |
 |
 |
 |
| Fig. 3 - Bulliard (1788) | Fig. 4 - Sowerby (1797) | Fig. 5 - Hornemann (1823) | Fig. 6 - Greville (1828) |
Figure 3 - Pierre Bulliard's illustration of Agaricus cretaceus [11] was described as corresponding well to the truth but being too exaggerated with its reddish tint and lacking the cottony character of the mushroom. This illustration appears to more closely resemble L. cepistipes rather than L. cretaceus and so Mattirolo's critique of the image suggests he was not collecting L. cepistipes mushrooms.
Figure 4 - James Sowerby's illustration [23] was not seen by Mattirolo personally but cited only based on the mention by other authors.
Figure 5 - Jens Wilken Hornemann's illustration of Agaricus cepaestipes from Floræ Danicæ Iconum [24] was described as 'leaving much to be desired'. Mattirolo notes that Elias Magnus Fries based his forma pumila upon this illustration. Mattirolo provides a citation of 'Flora danica (vol. X, fasc. XXVIII a XXX; tav. 1721, anno 1800)' for this illustration however tab 1721 is Stereocaulon incrustatum so this appears to be a citation error. Agaricus cepaestipes is found on tab 1798 of vol. 10, fasc. XXX but this was published in 1823 not 1800. This illustration is presumably the one that he meant to cite however as he said that it leaves much to be desired and indeed it does lacking in both detail and colour.
Figure 6 - Robert Kaye Greville's illustration of Agaricus cepaestipes [25] was not seen personally by Mattirolo but was examined by Pier Andrea Saccardo who said it corresponded well to the fresh specimens sent to him by Mattirolo.
 |
 |
 |
 |
 |
| Fig. 7 - Barla (1889) | Fig. 8 - Patouillard (1889) | Fig. 9 - Gillet (1874-1898) | Fig. 10 - Cooke (1881-1891) | Fig. 11 - Cooke (1881-1891) |
Figure 7 - Jean-Baptiste Barla's illustrations of Lepiota cepaestipes [26] found in figures 7-11 amongst several other species also regarded as Lepiota at the time do not appear to contain much detail to distinguish them from other white mushrooms. Mattirolo cites figures 5 and 6 of this compilation, which represent Lepiota rorulenta (now known as Leucocoprinus cepistipes var. rorulentus) which are said to correspond well to the species type.
Figure 8 - Narcisse Patouillard's illustration of Leucocoprinus cepaestipes [27] from 1889 is described by Mattirolo as 'recommendable, on the whole'. This illustration, with its distinctly scaly cap and stipe clearly does represent Leucocoprinus cretaceus rather than L. cepistipes.
Figure 9 - Claude-Casimir Gillet's illustration of Lepiota cepaestipes [28] is described by Mattirolo as being one of the best depictions. This illustration does resemble L. cretaceus so may support that being the species Mattirolo was collecting.
Figure 10 - Mordecai Cubitt Cooke's illustration of Agaricus (Lepiota) Cepaestipes var. cretaceus [29] is only described as 'excellent'. The base of the stipe in this illustration strongly resembles that of L. cretaceus. Cooke also illustrated Agaricus (Lepiota) Cepaestipes in this text (fig. 11) with white and yellow forms owing to the confusion between the different species. However Mattirolo does not cite this illustration and since it appears in the first volume of 'Illustrations of British Fungi' 1881-1883 as opposed to the volume VIII supplement published 1889-1891 it is not clear if he also saw and compared this one.
The text does not make it perfectly clear if Mattirolo actually did eat L. cretaceus but his list of illustrations do point to that being the species he was observing. Mattirolo was never able to successfully cultivate any of the Leucocoprinus species he was studying due to contamination issues and so his observations were made only on specimens that grew in the greenhouses, where the mushrooms had been introduced with tropical plants. Interestingly he did not observe sclerotia in this species and stated that it had none however when it is cultivated the sclerotia are abundantly noticeable and seem to always appear before the mushrooms.
5. Cultivating Leucocoprinus cretaceus
The bulk of the information on culturing L. cretaceus for this experiment is presented in 'A preliminary investigation of sclerotia in Leucocoprinus cretaceus' as it was the growth of the sclerotia that was first noticed. The first mushrooms which were observed began growing only after sclerotia were harvested for examination though subsequent observations have since been made of numerous specimens grown on various substrates. In sealed jars at room temperature or with heating elements the mushrooms appear to grow readily and easily on a variety of substrates and have a rapid rate of growth such that regularly harvesting them becomes necessary.
The duration of time between immature primordia developing and the mushrooms maturing to the point at which the cap opens appears quite variable and dependant on environmental conditions with heat and humidity accelerating the process. When conditions are less than optimal the primordia appear to develop more slowly and sometimes seem to stall in their growth to grow more or abort later. Once the cap opens however the mushrooms only last for around two days before deteriorating so the window of time in which to harvest them is relatively short. Likewise the stems of the mushrooms, if kept in the fridge will not last more than a couple days before withering and darkening so dehydration is necessary for storage. The caps and stems dry very readily however and can be preserved even if just left to dry at room temperature without the use of a dehydrator.
In nature Leucocoprinus cretaceus is a very versatile saprotroph and is observed growing on trees, fallen wood, amongst woodchips, from termite tests, in plant pots, from compost piles or in compost bins or just straight from the ground. There are also numerous observations of it growing in houses from walls, floors, furniture and carpets (some of which are collected here) and whilst it would be inadvisable to eat any mushrooms grown on such structures it does point to a real versatility in their growth habits.
It is therefore likely that they will perform well on many different substrates in captivity and whilst it will take much more testing to determine the optimal substrate they have so far grown well on basically everything that has been inoculated. Potting soil and coir perform well and the addition of leaf matter from used tea or fallen leaves from chillies and tomatoes appears to greatly improve growth though these nutritious substrates also result in the production of far greater numbers of sclerotia.
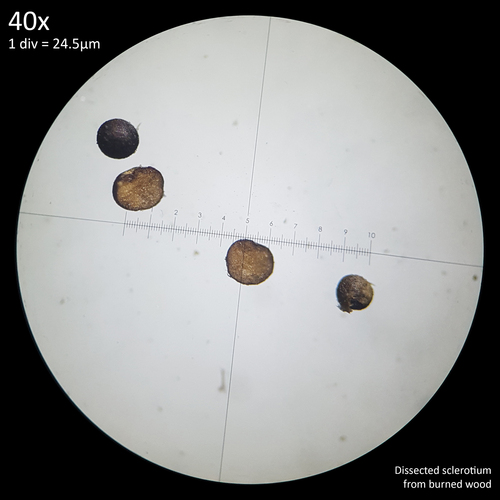
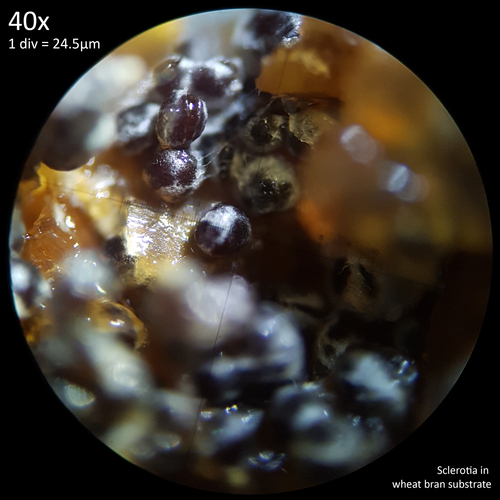
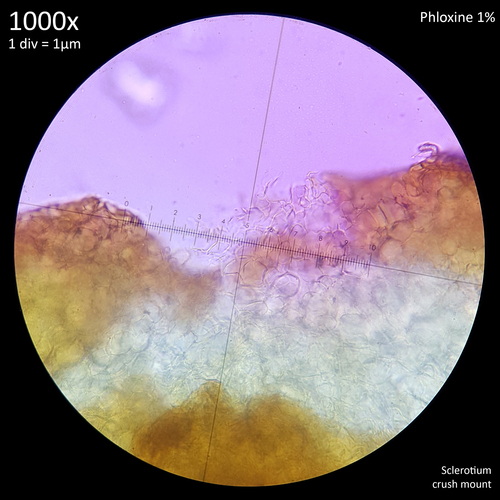
Note that is it important to recognise the role sclerotia play in the growth cycle and the edibility of these hard, sand grain like structures has not been assessed with only the mushrooms consumed. In each case detailed below in which mushrooms were consumed time was first taken to remove the substrate and sclerotia from the base. Sclerotia can stick to the exterior of the mushrooms and in some cases are embedded in the surface of the stem base although dissection has so far not revealed any deeper inside the mushrooms. As the sclerotia are very hard and have shown some resistance to both heat and chemicals it is not yet clear how digestible they would be or if cooking would be reliable at rendering them inert. An additional concern is that the sclerotia appear to serve as a distribution method with new mycelium being able to grow very easily and rapidly from the displaced sclerotia when placed in a humid environment. Even if they are stuck to the inside of a glass jar and not in contact with the substrate mycelium will grow out from them. The sclerotia are the perfect size to get stuck in the gum line or in a gap between teeth and whilst it seems unlikely that they would manage to grow in this environment without being suppressed by the immune system it is probably best to avoid putting this to the test until more is known. Unlike plant seeds which would absorb water and soften over time if lodged between teeth the sclerotia appear to remain hard and unchanged even if soaked in water for a prolonged duration.
5.1 Observations
 |
| Fig. 12 - First mushroom harvested |
On 11/08/23 one mushroom weighing ~5.4g was harvested from a colonised jar of enriched rice used for the sclerotia experiment. The substrate was comprised of 75g brown rice, 0.75g yeast extract, 0.75g soluble starch (potato), 0.75g gypsum, 20g hardwood fuel pellets (oak), 105ml water in a tall, 500ml mason jar, filled about half full with a 0.3µm PTFE filter disc on the lid for airflow. The jar was inoculated from colonised agar 45 days prior to harvesting the mushroom but was only initially intended to test for contamination since this substrate had been prepared and sterilised for a previous experiment some months prior but went unused and subsequently appeared dry. The excellent growth therefore came as a surprise. The day by day growth documenting the development of the first primordia after harvesting sclerotia is displayed in figures 19a-19h in 'A preliminary investigation of sclerotia in Leucocoprinus cretaceus'.
The cap had opened overnight and had developed a brownish yellow centre. Upon opening the jar to harvest, a strong and pungent, though not unpleasant fungal smell was immediately noticeable however the mushroom itself did not smell so distinctly. The mature mushroom has a pleasant fungal smell but it is not overly noticeable compared to the smell of the mycelium and the younger pins which are far stronger. The immature mushrooms individually do not smell that strongly but when clustered together it is quite noticeable. When the stem of the mature mushroom was cut in half the smell was slightly stronger and more pleasant. The base of the stem was removed and kept for study as it came away with some substrate and many sclerotia clustered around it. After leaving it in the open air for a couple hours the smell seemed faintly similar to that of cooked Grifola frondosa but still with a slight chemical undertone. This however was only noticed after cooking the mushroom and so may have been biased by the whole house smelling like this. When left longer to dry in the open air there was no smell to the stem or base with the mycelium and sclerotia on the substrate. A section of the cap amounting to approximately a quarter of the total cap was left to spore print in a sealed tub and remained left sealed for some days after. Upon opening this no significant smell was noted.
The taste of the mushroom raw was likewise not unpleasant though not overly distinctive. After spitting it out the aftertaste was more noticeable and had a slightly chemical or floral taste which was hard to place. The taste of the cap was perhaps slightly less pleasant than that of the stem with a more noticeable chemical taste however it is the texture of the cap that makes it less desirable to taste as it feels like rolling a piece of cotton wool around in your mouth. It is unsubstantial and cottony in texture much like the description found in many Leucocoprinus species.
The cap falls apart relatively easily when handled and is fairly fragile with thin flesh whereas the stem is firm and rigid. The base of the stem is solid and quite firm, not compressing much at all when squeezed however when a piece was left for some hours it became soft and easily compressed. Higher up the stem is pithy inside, though still quite firm and felt as though it could be too tough to eat. On squeezing the stem slightly it did not yield greatly but a yellowish or brownish colour similar to the centre of the cap is produced, however the reaction is not instant and not very distinctive. This colouration was already present in some places of the stem before it was harvested but less noticeable beneath the powdery white scales, which easily displace and stain the fingers on touching. So squeezing it also removed the scales and increased the visibility of this colouration. When the base of the stem was cut no discolouration was noted but upon cutting the thinner stem higher up a yellowing occurred on the outer surface but stopped 1mm or less into the pithy inner flesh, which did not discolour. A section of this inner flesh was tested with KOH to see if this would accelerate discolouration but it did not. No reaction to KOH or FeSO4 was observed on any part of the mushroom.
5.2 Cooking
Please note that consuming this mushroom was only undertaken after extensive reading of the taxonomic history and literature on this species and others in order to determine that it would be safe. Toxic species in the Agaricaceae family are generally only gastrointestinal in nature, if Lepiota species are excluded and taken as belonging to Verrucosporaceae (as iNaturalist has them in opposition to Species Fungorum and Mycobank which still classify them as Agaricaceae). There are some case reports suggesting fatal poisoning by Chlorophyllum molybdites in small children and dogs but no such cases have been reported in adults. I have seen no fatal reports for anything else in the family and therefore it did not seem likely that there would be any risk of serious toxicity. The multiple mentions of edibility in the literature for this species also served to dispel fears but only after sufficiently interrogating each source to try and confirm which species they were actually talking about - a vital step given the numerous confusions found in the taxonomy. The suggestions of edibility would not have been enough to give me confidence to try eating it if there was anything seriously dangerous in the family.
The species identity was confirmed via microscopy as well as observations of the intact mushrooms whilst growing them. In the wild L. cretaceus can lose many of its distinctive identification features due to rain and damage as can be seen here. There are numerous Leucocoprinus and Leucoagaricus species for which it could be mistaken and for which the edibility is still unknown. More seriously it would not be inconceivable for inexperienced foragers to confuse this species with some of the deadly Lepiota or Amanita species. Species in Amanita Sect. Roanokenses get mistaken for L. cretaceus regularly and some of those can be seriously toxic.
Whilst consuming the cultured mushrooms has not caused me any issues I would not suggest that this species is suitable for foraging due to the short period of time in which they are fresh and the risk of confusion.
 |
 |
 |
|---|---|---|
| Fig. 13a - Before cooking | Fig. 13b - During cooking | Fig. 13c - After cooking |
An hour after harvesting, 1g of cap and 2.4g of stem was cooked by sauteing in a pan with butter, without any seasoning. This amounted to most of the cap besides a section kept to study and most of the stem besides the very bottom of the base and some small pieces that were tested.
After browning the butter slightly the cooking time was approximately 5 minutes on a low heat in a stone coated pan. The mushroom was initially divided into three pieces - the cap, middle of stem and base of stem but as the base was thick and did not shrivel when cooked it was cut in half during cooking to brown both sides. Surprisingly the mushroom did not shrivel and diminish as much as some others do when cooked and it browned quickly. The cap reduced in size slightly but the stem pieces retained their consistency well. The smell during cooking was pleasant and persisted in the house for a few hours afterwards being somewhat reminiscent of the smell of fried chicken or similarly chicken-like as the smell of cooking chicken of the woods and hen of the woods, though the smell of the butter itself may have influenced this comparison.
The thinner, middle piece of the stem was tasted first and was surprisingly delicious. It was meaty and flavourful and somewhat reminiscent of chicken of the woods with a texture that was neither too tough, crispy, chewy, soggy or insubstantial. It is close to or slightly stronger than Pioppino, Cyclocybe aegerita in taste but with a slightly firmer, meatier texture which made it more pleasant. The cap was not as great as it had browned and cooked more than the stem and so had a slightly burnt taste, a little like burnt toast - though it did not look as if it was burnt and was not crispy. It was not unpleasant as such but the texture was still cottony and relatively insubstantial. A slight hint of the chemical taste that the cap possessed when raw was noticeable but was not enough to make it unpleasant. It may be necessary to start cooking the stems first and add the caps after a minute or two in order to prevent overcooking, or better yet harvest younger mushrooms before the cap opens. The two pieces of the base of the stem did not taste as strong as the middle section though were still very pleasant. With their greater girth they had not cooked through as much even when cut in half so were softer inside and similar in taste and consistency to cooked chicken of the woods.
Mattirolo makes the comparison to Pelliccione - Macrolepiota procera but from his description it is not certain if he is comparing the taste or only the appearance of the gills. As I have only ever found Macrolepiota procera in protected woodland on a nature reserve and hence have not picked and eaten them I cannot make this comparison however I do know that the stems are often regarded as too tough to eat and are discarded. So it interesting that with Leucocoprinus cretaceus the stem texture is actually very pleasant and frankly perfect for consumption. I am not a huge fan of the texture of many cooked mushrooms which can often be too soggy or overly crispy but I thoroughly enjoyed sampling this mushroom. It seemed sensible to consume only a small amount to test for any gastrointestinal symptoms but frankly if more than one had grown I would have been tempted to eat them all since the taste left me wanting more. No symptoms occurred as a result of eating this mushroom and there was no reaction to alcohol.
The best time to harvest these for consumption may be before the cap opens or even before the stem has substantially grown. After harvesting and eating this mushroom the immature mushrooms remaining in the jar aborted and gradually diminished however many new primordia grew afterwards. Aborted mushrooms appear to desiccate themselves quite effectively even whilst embedded in a moist substrate.
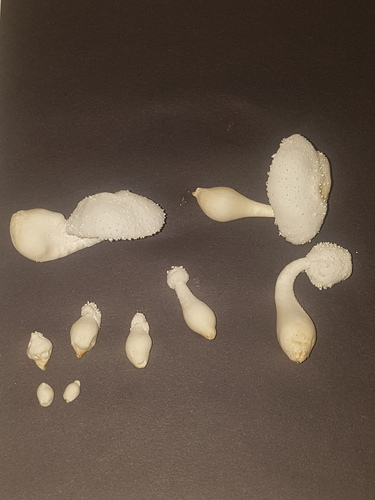 |
 |
 |
|---|---|---|
| Fig. 14a - After harvesting | Fig. 14b - After cutting | Fig. 14c - During cooking |
On 15/08/23 more mushrooms were harvested from the same jar with a total fresh weight of 10.78g. This represented one large mature mushroom with the cap open, one of a similar size with a closed cap, a smaller less mature one with a closed cap, two conjoined mushrooms with the base and cap attached but individual stems noticeable and then a few small, undeveloped pins that came attached to others.
One mature mushroom cap and stem was sauteed in sunflower oil for ~2-3 minutes without seasoning. The whole stem was cut down the middle from top to bottom and cooked in two pieces and the cap was cut in two. The stem was again pleasant, though was better in butter and the cap still had the slight burnt taste. It was not unpleasant but again reinforced the thought that they are probably best harvested before the cap opens.
The remaining mushrooms were sauteed in butter for 4-5 minutes without seasoning. The unopened caps proved far better than the open ones and were quite reasonable to eat. In both taste and texture they were preferable. The stems were again very pleasant with the thinner portion becoming more crisped than the base, which had soft flesh within. The small immature mushrooms were cut in half down the middle or cooked whole. Subsequently they cooked faster than the larger ones and browned more but did not burn. The taste was not substantially different to the mature stems but no off taste or texture from the immature cap was noticed.
 |
 |
|---|---|
| Fig. 15a - After harvesting | Fig. 15b - After cutting |
On 01/09/23 two dozen mushrooms and some immature pins weighing a total of 35.87g fresh were cooked and consumed. Mushrooms were harvested from several jars over a two day period with some stored in the fridge overnight. They were grown in jars containing rice, potting soil or a potting soil and coir mix. Some smaller ones were grown in a small container full of only wheat bran and water which had been intended to test sclerotia growth but resulted in mushrooms nonetheless.
The mushrooms were divided into two portions with the first sauteed in just butter and the second sauteed in butter and seasoned with black pepper. Each portion contained a mix of mushroom caps and stems in different stages of maturity. The stems were cut into sections and added to the pan first with the caps added a minute or two later once the stems had started to brown. The caps of the young mushrooms that had either yet to open or only recently opened were vastly preferable to the older ones and when cooked with pepper actually tasted even better than the stems, though perhaps this was just the result of them absorbing a great deal of butter. Some that were overcooked or older still had a slight unpleasant bitterness and the texture was still not ideal with any of the ones that had expanded though they were still tolerable and not a big problem. With caps that remained closed or had only just started to open there was a meatier texture and they cooked quite well. As a result the taste was surprisingly variable between the different parts at different stages of maturity.
 |
 |
 |
|---|---|---|
| Fig. 15c - During cooking | Fig. 15d - During cooking | Fig. 15e - After cooking |
No issues arose after eating this larger serving. The interior flesh in some thicker pieces of stem that had only been bisected was not perfectly cooked and so it is probably better to cut into quarters. The ideal time for harvesting is probably when pins have started to enlarge but before the caps have begun to open. If mature caps are to be cooked they require less time than the stems so as to be palatable. One mushroom that formed had a stem that was wider for a greater portion of the length and grew to around finger size with a cap that was small and unopened and this proved ideal for consumption. Similar has been observed multiple times but it is not yet clear if this is the result of chance environmental conditions or whether this trait can be reproduced via cloning.
Experimentation with consumption of the mushrooms was paused at this time due to unusual symptoms of nasal congestion which occurred and persisted in the weeks following. It was severe enough to cause constant sneezing and at times a complete loss of smell such that it was not possible to distinguish isopropyl alcohol or vinegar from water by smelling a tub held right under the nose. Whilst it seemed unlikely that the mushrooms were responsible for these peculiar symptoms they could not be ruled out as the cause and so further consumption had to be halted. The very strong and unusual smell of the substrate and sclerotia was also considered as the cause and general hypochondria ensued due to a family history of nasal polyps causing anosmia.
However the culprit appeared to be contamination of the grow room with mites, probably Tyrophagus puterescents which in some places had left significant quantities of dusty material behind which was triggering a major allergic response. Symptoms quickly disappeared when walking outside and escalated when entering the grow room. Some of the mushrooms consumed were also collected from substrates that were likely contaminated by the mites. Once the infested material was removed, the debris cleaned up and more suitably mite-proof containers were employed to prevent further infestation the allergic response diminished. After the symptoms alleviated they did reoccur briefly on a couple of occasions after emptying mite contaminated jars into the compost and so it would appear that exposure to the mite debris was the trigger for the symptoms. During this hiatus any mushrooms harvested were dried for later and on a few occasions some dried stems were added to pizza without issue.
 |
 |
 |
 |
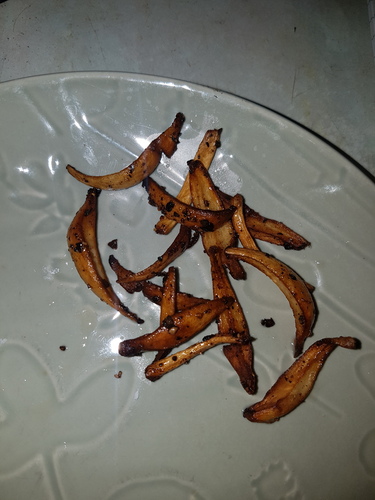 |
|---|---|---|---|---|
| Fig. 16a - 00:36 - Before cooking | Fig. 16b - 00:37 - Cooking start | Fig. 16c - 00:38 - Cooking | Fig. 16d - 00:39 - Cooking | Fig. 16e - 00:40 - After cooking |
On 31/12/23 three large mushrooms grown on a soil substrate were picked within hours of the caps opening and weighed a total of 20.5g. The caps were removed for spore printing and the masses of sclerotia at the stem base were removed leaving 15g of stems with bases that measured 14.5, 16 and 18mm thick. The stems were cut half way up to separate the thinner top portion of the stem from the thicker base and then the bases were quartered down the centre. All the stem material was then sauteed in sunflower oil with black pepper for approximately 4 minutes. Figures 16a-16e show the browning during cooking with photos taken 1 minute apart. The cooked stems had a pleasant taste and texture with quartering them allowing them to better cook through. No symptoms occurred after consuming them.
The following day a further 7.7g of stems were cooked similarly but with the addition of ground Nigella sativa seeds and Cajun spices which further improved the already good flavour. No symptoms occurred. On 05/01/24 one especially large mushroom was picked which had a total weight of 13.1g of which 8.5g was the stem which had a thickness of 18-19mm at the base. This was basted in sauce and roasted in the oven resulting in a consistency similar to potato wedges with a crispy exterior and softer middle. The next day a 7g mushroom was picked and 4.1g of stem was roasted on a baking tray in sunflower oil with potato wedges. It cooked well and became crispy and firm in around half the time that the potatoes took.
On 21/01/24 dried mushrooms were soaked in water to rehydrate with five dried mushroom stems weighing a total of 1g placed in a cup with approximately 100ml of cold tap water. The stems floated but rehydrated quite readily however they became more soggy and lacked the firmness of the fresh stems. The thick stem bases rehydrated substantially and enlarged to something similar to their original size in three of the five mushrooms but were quite water logged and soggy to the touch. The middle portion of the stem remained quite firm and inflexible similar to when fresh but the apex of the stem was very soggy. Two of the stems remained somewhat shrivelled and had a browner colouration but felt a bit firmer than the others. These may have been specimens that were picked during a different state of maturity or air dried as opposed to placed in the dehydrator. This was not recorded as all dried mushrooms were simply kept together in an air tight container.
The stems were removed from the water after around 10 minutes and weighed 4.2g total which is probably similar to the weight when fresh since these were all small specimens. The sogginess made them awkward to cut and so the smallest were cooked whole. After sauteing in sunflower oil and black pepper for approximately 4 minutes the sogginess was not an issue and they crisped up well. The taste and texture was good and similar to when fresh though perhaps slightly preferable with fresh mushrooms. The dried mushrooms have a very pleasant savoury smell which is noticeable when opening the container but after soaking and cooking this was not noticeable. If larger specimens are to be dried for later cooking it will probably be optimal to cut them first.
5.3 Notes on the Smell
On 30/08/23 an over-mature mushroom was harvested from a 500ml jar with a substrate comprised of 50g potting soil, 15g coco coir, 10g wheat bran and 110ml water. The addition of wheat bran had resulted in faster colonisation in this jar as opposed to the ones with just soil and coir mixes however was not necessary to result in fruiting with mushrooms ultimately growing in all jars except for the one that was comprised of only coir.
The jar was opened and placed into a tall, clear plastic food storage container with four 0.3 µm filters covering 7mm holes to provide fresh air exchange. This did not allow much moisture to escape however and so condensation quickly built up resulting in water pooling on the surface of the substrate and in the bottom of the fruiting chamber. No contamination was evident but yellowish metabolites were visible in the liquid on the top of the substrate. When the jar was introduced into the fruiting chamber immature mushrooms were already present measuring a few cm in length. However upon dropping the jar down into the chamber the immature, unopened caps of some of these mushrooms simply fell off and even the undamaged ones aborted.
 |
 |
 |
 |
 |
 |
| Fig. 17a - 26/08 22:30 | Fig. 17b - 27/08 18:00 | Fig. 17c - 27/08 22:30 | Fig. 17d - 28/08 12:40 | Fig. 17e - 29/08 14:00 | Fig. 17f - 30/08 14:00 |
Figures 17a-17f shows the growth of this solitary mushroom over the course of four days, the second immature mushroom attached at the base to the larger one aborted and did not grow any more after the larger one began to develop. The cap did not expand and flatten as significantly as some other specimens but remained open for at least 51 hours before ultimately collapsing (additional photos were taken that have not been uploaded due to quality issues caused by the condensation). The window of time in which to harvest the mushrooms once the cap opens would appear to be around two days and this has likewise been observed in other jars where no mushrooms were harvested resulted in them deteriorating and ultimately smelling rather off putting.
The last photo in this series shows the mushroom as it was harvested. It appeared as if the cap had detached entirely but in fact the stem had simply bent and it was still in one piece. This over-mature mushroom had a far more pronounced and unpleasant odour with the cap having a somewhat floral, but still unpleasant, scent and a much stronger and more unpleasant smell to the base of the stem and substrate which did indeed border on nauseating. The smell was off-putting enough to suggest that it would be no good to eat and so this mushroom was not cooked and consumed. After leaving it to sit in the open air for a couple of hours the floral smell was no longer present and all that remained was the unpleasant, chemical odour which if anything seemed stronger.
Despite the smell, the taste of the raw upper stem was not wholly unpleasant. It had an initial taste similar to the unpleasant smell with perhaps a very slight bitterness but it was not significant and it quickly gave way to a generally mushroomy taste that was reminiscent of the cooked taste of this mushroom. The cap however had a more unpleasant taste with more chemical overtones similar to the smell. The texture of the cap remained cottony and unappetising and whilst the stem had softened and become more water logged it still retained a reasonable texture, though squeezing it resulted in it yielding far easier than when fresh.
After tasting the mushroom and then smelling it again the smell appeared different. The cap smell seemed more pleasant and reminiscent of the taste but the base of the mushroom with the colonised substrate and sclerotia still attached retained the unpleasant smell. It is unclear if tasting the mushroom impacted the perception of the smell or if further maturation had occurred in the 20-30 minutes after tasting it.
The smell was compared against dried samples harvested on 11/08/23 which comprised of a small piece of cap and gills, a small section of the upper stem, the very bottom portion of the stem with some brown rice substrate and sclerotia attached and a few small primordia dissected on a slide. These are stored together in an air tight food grade plastic container and the smell was sampled by opening the container but not testing individual parts. The overall smell is very pleasant with a hint of the typical savoury smell found in many dried mushrooms but with a sweet, almost chocolate or toffee like smell on top of that.
This smell is not wholly dissimilar to the over-mature mushroom and when compared one after the other the same hints of fragrance can be noted. It appears that the more unpleasant smells come through as the mushroom ages and begins to deteriorate. This over-mature mushroom and another left to wither for even longer in another jar were stored in separate food grade plastic containers with the lids ajar to allow some drying. No noticeable decomposition occurred and they did slowly dry. They were sampled day by day by removing the lid and smelling the air trapped inside and the smell seemed more unpleasant each time, though not necessarily always the same perhaps due to the amount of time allowed between each sample. On some occasions the smell was strong, pungent and quite off putting but on others was simply unpleasant and a bit chemical in nature. It is different to the smell found in the substrate or immature mushrooms however which is not so unpleasant but more pungent.
When a small piece of the soil substrate was examined under the microscope and cut in half to collect sclerotia for culturing, a strong smell was immediately noticeable, even whilst wearing a mask to avoid contamination. This smell isn't especially pleasant or unpleasant but simply comes across as chemical and a may be a little overpowering in larger samples. This piece of the substrate had been removed from the container an hour or two earlier and had sat in the open air but even so, upon exposing the interior the same pungent smell was noticeable as when the jar was opened initially. When a small piece of soil substrate with sclerotia, immature and aborted pins weighing 1g total was left out on the desk in a small room with little to no airflow or ventilation there was a noticeable smell upon entering the room.
This helps explain the variation noted in the descriptions of smell given by different authors. The mushrooms have a stronger, more pleasant smell when immature but this is masked by the far stronger, chemical smell of the myceliated substrate which clings to the base. When more mature and in their prime for picking the smell is not as significant and may not even be noticeable. It could easily vary from being described as generally mushroomy, pleasant but indistinct or simply having no smell. When over mature the smell becomes unpleasant and quite off putting with an initial floral smell that is lost to that unpleasant odour. Yet as they are left to dry the smell ultimately becomes more pleasant, appetising even.
The range of smells that are observable through cultivating this mushroom are really quite fascinating and whilst this description may go some way to explaining the variable descriptions given by others, it seems paltry compared to the experience as I cannot find the words to properly describe the smell at each stage of development.
6. Cultivation of tropical mushrooms as a matter of food security
This experiment in cultivating and consuming Leucocoprinus cretaceus was driven in part by my awareness of climate change and the concerns I have for the impact it poses to food supplies. It seemed worth exploring whether a mushroom that thrives in high temperatures and grows on readily available substrates could be grown for food.
Some issues that may potentially be faced with large scale cultivation are posed by the smell of the substrate, which is so surprisingly strong in just a small jar that it is hard to imagine what a warehouse sized grow would smell like. It could make for quite an overpowering environment to work in and it seems probable that the smell would lure in insects and pests. On the plus side however the spore production does appear to be far lower than with some commonly cultivated species and so health risks faced by the inhalation of large quantities of spores are probably reduced.
The presence of the sclerotia would also need to be addressed either by determining that they are safe to consume or formulating a removal method. With commercial cultivation of Agaricus bisporus it is common to simply cut the stem above the base to lose the bottom portion of the stem with the soil substrate that clings to it. This would be less than optimal for L. cretaceus as this is where the bulk of the flesh is found. Even if sclerotia do not pose a risk to consumption they would be less than ideal to cook as when heated on a metal surface they can be flung into the air and get lost. If they are not sufficiently damaged by the heat as to be rendered sterile then this could easily result in the mushrooms growing from floorboards or the base of skirting boards if water is spilled or otherwise leaks. Numerous observations on iNaturalist show the mushroom growing from beneath skirting boards or door frames and it seems possible that this may be due to sclerotia washing in with rainfall or floodwater. Since sclerotia production appears to be greatly reduced or entirely absent on substrates comprised of wood it may be viable to effectively eliminate sclerotia from the mushrooms with a top layer of wood on the substrate.
Some sclerotia would almost certainly survive in spent substrate so if this was sold as compost these mushrooms would become far more common and spread wherever this compost was used, similar to how L. birnbaumii spreads via potting soil and potted plants. It is probable that L. cretaceus already spreads in this manner given the numerous observations worldwide of it in potted plants and planters filled with commercial soil. However these observations are far, far fewer in number than those of L. birnbaumii and so for reasons that are not yet clear it would appear that L. cretaceus may be less proficient at spreading in this manner. Alternatively it could simply be that L. cretaceus has not been introduced into the potting soil/potted plant distribution network in such a widespread fashion.
Whilst it may be desirable to avoid sclerotia on the mushrooms destined for consumption their production could have other useful functionality. The carbon/nitrogen composition of the sclerotia is as yet unknown but it is probable that they are mostly comprised of carbon. As they are produced in enormous numbers such that they represent a sizeable weight of the spent substrate they could have a role in carbon sequestration. The sclerotia are very hard, durable and self-desiccate naturally and these traits appear to make them resistant to colonisation by mold or consumption by insects and mites. As such carbon locked away in sclerotia could be expected to remain there for a prolonged time, though mycelial growth may occur if they become wet. If sclerotia were treated as a sand-like aggregate and bonded together with a suitable resin they might make a viable construction material whilst also locking away carbon.
Leucocoprinus cretaceus does not appear to have been considered for cultivation before since the edibility of this species seems to have gone unnoticed. A 2021 review of the world's edible mushroom species lists Leucocoprinus birnbaumii as poisonous and Lc. cepistipes as conditionally edible and also includes Leucoagaricus americanus as conditionally edible, La. badhamii as poisonous and La. leucothites as edible.[30] Leucocoprinus cretaceus is not mentioned in this paper nor is it, or any Leucocoprinus species mentioned in a 2014 study on cultivating wild tropical mushrooms.[31]
However in Charles McIlvaine and Robert K. Macadam's 1900 'Toadstools, Mushrooms, Fungi, Edible and Poisonous: One Thousand American Fungi' L. cepaestipes is listed as edible and enjoyable when cooked in either its white or yellow form. Agaricus cretaceus is likewise listed as edible though again it is unclear which species this refers to.[32]
Nancy Smith Weber and Alexander H. Smith's 1985 'A Field Guide to Southern Mushrooms' also mentions an acquaintance of theirs consuming Leucocoprinus luteus several times without issue.[10] As recent sequences seem to suggest numerous yellow species which get identified as Leucocoprinus birnbaumii it is unclear which was consumed. It seems possible that reports on the toxicity of this species may be based on raw consumption with toxins that could be denatured when cooked. Alternatively it could be similar to other species in the Agaricaceae family which cause reactions for some people but not others. This too would also need to be ascertained for Leucocoprinus cretaceus by more people sampling them.
A 2019 review of mushroom cultivation in Bangladesh which focuses especially on Pleurotus species suggests that consumption of mushrooms may help address the malnutrition that is found to some degree in half of the population of the country. Based on yields produced, the optimal growing season for Pleurotus species was determined to be December-February with decreased growth from March onwards, moderate growth in April-July and minimum growth from August-October, increasing from November onwards. High summer temperatures are noted as one of the problems encountered with commercial cultivation.[33] A 2012 study on the cultivation of Agaricus bisporus in Bangladesh lists the problems faced by a sample of 30 producers with fly and cockroach issues being faced by 67% and contamination issues faced by 37%. Hot temperatures were faced by 40% of producers and amongst the technical issues that arose air conditioner problems were encountered by 50% and electricity by 60%.[34]
It seems probable that cultivation of L. cretaceus could address the issues faced in regards to heat and grow during the summer months in Bangladesh easily without air conditioning. iNaturalist observations for Leucocoprinus cretaceus in Bangladesh are few and temperature data from the region is not always available. Observation 105564290 by @biodiversityofbangladesh shows a nice flush of mushrooms in multiple stages of maturity spread across the floor of a forest near Khulna in Bangladesh on the 11th of June, 2021. However timeanddate.com is missing the weather data for a lot of Bangladesh during much of that year and so it isn't clear what the environmental conditions were and at best it can be approximated based on the 33°C recorded in Kolkata that day. Observations from India are greater in number and there are several from West Bengal for which temperature data is available.
Observation 172145361 by @wild_wild_nature was recorded on the 10/06/23 fruiting on wood near Kolkata. Time and date records a high temperature of 34°C and a low of 29°C on this day with similar highs and lows in the days before and after.
Observation 160716481 by @puspak_roy from 09/05/23 shows a moderately large flush fruiting on soil, compost or possibly manure in Bardhaman University. Some of the pins appear to be aborting and some larger mushrooms seem dry though several look like they are developing normally. Time and date records a high temperature of 42°C at midday and a low of 29°C this day with a high of 42°C on the previous day, 40°C before that and temperatures in the mid to high 30s in the previous week. Leucocoprinus cretaceus can grow to full maturity in just a few days so this suggests the mushrooms were able to develop despite these very high temperatures.
Observation 171760762 by @aniruddha_singhamahapatra from 08/07/23 shows a large flush of fully developed mushrooms growing on soil or woody debris beneath a forest canopy in the district of Bankura. Time and date records a high of 33°C and a low of 27°C this day with the same temperatures the day before, some rain in the preceding three days and temperatures in the mid to high 30s in the week prior.
Observation 95750750 by @banerjeed from 20/06/23 shows immature pins forming in a plant pot or planter in Asansol. Time and date records a high of 33°C and a low of 25°C this day with temperatures in the high 20s the week prior.
Observation 82690927 by @earthanki from 12/06/21 shows a single well developed specimen in a plant pot that was noted as growing in just one afternoon during the rainy season in Sonitpur, Assam, India. Time and date records a high of 33°C and a low of 27°C this day with a high of 29°C and rain the day before and rain and storms in the week prior with temperatures in the high 20s to low 30s.
A total of 200 observations of Leucocoprinus cretaceus worldwide from iNaturalist with the temperature data were collatedhere revealing many were growing at temperatures above 30°C with the highest temperature of 42°C. Whilst they will grow adequately at room temperatures in the mid 20s it would appear that their temperature tolerance extends far beyond this giving them potential for cultivation in hot climates.
7. Conclusion
Leucocoprinus cretaceus appears to be a perfectly edible mushroom with no side effects documented thus far however as the sample size for this experiment was only two people it would be prudent for others to sample this species and confirm that they have no reactions either.
Whilst these edibility tests cannot eliminate the possibility of longer term side effects these do not seem likely given that toxicity in members of the Agaricaceae family are gastrointestinal in nature with symptoms that emerge quickly rather than ones which bioaccumulate. The edibility of the sclerotia still needs to be explored as whilst it seems unlikely that they would be toxic or pose any health risk, the subject of sclerotia in Leucocoprinus species has not been sufficiently documented and so little is known about them. Given their significant hardness and resistance to chemicals it seems possible that they are not digestible and probable that they are best avoided. However the sclerotia are relatively easy to remove from the mushrooms with most dislodging readily when brushed off. One solution to reducing the amount of sclerotia present stuck to the base of the mushroom is adding a layer of wood chips to the top surface of the substrate. This seems to result in the production of fewer sclerotia compared to higher nutrient substrates like wheat bran or rice and is easier to clean from the mushrooms than soil.
The species is very easy to grow, performs well on numerous subtrates and will grow well simply on potting soil or soil and coir mixes that enable higher water content. Excellent growth is also observed on plant material like leaves and stems and so it may have potential for composting garden waste. It fruits so readily and quickly that most substrate tests resulted in mushrooms growing in the jar, often before fully colonising the substrate, meanwhile other Leucocoprinus species stored beside them in similar jars of substrate have yet to fruit at all. As yet however mushroom growth is sporadic and somewhat unpredictable with jars moved into fruiting chambers with heating sitting idle for prolonged periods meanwhile neglected jars left at room temperature may intermittently fruit when least expected. As such more experimentation will be required to assess the optimal fruiting conditions regarding temperature, humidity, air flow and lighting in order to improve yields and achieve predictable growth.